Chicken soup may be good for the soul and the sniffles. Now scientists are enlisting chickens to help make much stronger medicine – including potential treatments for cancer.
"In the chicken world, we're all very excited. It won't happen this year, but it will happen," said Mary Delany, professor and chair of the UC Davis Department of Animal Science.
One of the world's foremost avian geneticists, Delany is forecasting the day when producing sophisticated anti-cancer drugs will be a matter of cracking eggs, each egg holding milligram-quantities of a desired agent. The technology would enable pharmaceutical companies to farm human proteins of therapeutic value on an industrial scale, more cheaply and efficiently than currently possible.
"A typical commercial hen house might have 40,000 hens that each lay six eggs a week," Delany noted. "That's 240,000 eggs a week."
Germ of an idea
In the prestigious British journal Nature last summer, scientists at a small Bay-Area biotech company, Origen Therapeutics, Inc., reported a major advance in avian transgenics – one that, in principle, should enable scientists to make any genetic modification desired to the chicken genome. Delany collaborated on the project and is an author of the Nature paper.
Adding a jellyfish gene
The team's advance involved several feats: isolating and successfully culturing primordial germ cells from the blood of chicken embryos, modifying the cells by adding a jellyfish gene for fluorescence, and then introducing these transgenic cells into the bloodstream of new chicken embryos at precisely the right stage of embryonic development.
The transgenic germ cells migrated to the gonads of the embryos, where they matured, as germ cells are intended, into sperm or egg cells as the embryos grew into chicks. After hatching, males were reared to maturity and crossed with nontransgenic females.
Some of the resulting offspring carried the jellyfish gene. The experiment was the first demonstration in any species that primordial germ cells can be immortalized, or grown indefinitely, in culture, and the first demonstration that the cells can be genetically manipulated while retaining their ability to mature into viable sperm and eggs that can pass transgenic material to a new generation.
For the birds
The achievement opens the possibility that instead of altering chickens to express a jellyfish protein, scientists will be able to engineer the birds to produce a human monoclonal antibody or other therapeutic protein. Indeed, Origen scientists have already shown in separate experiments that a transgenic protein can be expressed in the whites of chicken eggs.
"They have made an incredible contribution," Delany said of her Origen collaborators.
Delany's own role was pivotal. Her group at UC Davis screened the cultured germ cells for genome organization and stability, making certain that only chromosomally stable cells were used in the project.
"Dr. Delany brought her internationally recognized expertise in avian cytogenetics to the analysis of primordial germ cells that had been in culture for extended periods," said Robert Etches, former vice president of research at Origen Therapeutics. "The combination of in-house expertise and world-class support from the Delany laboratory allowed Origen to complete the technology quickly and efficiently."
The new approach has particular potential for production of monoclonal antibodies used in cancer treatment, including Herceptin for breast cancer and Rituxan for lymphoma. Such drugs are now grown in Chinese hamster ovary cells and then turned into drugs at bioreactor plants, a costly and timeconsuming process.
Crucial support
Delany has been studying chickens and their genes since first joining UC Davis in 1995. But it was a modest Institutional Research Grant from the American Cancer Society, matched by funds from the UC Davis School of Medicine, that fixed her focus on cancer.
For research scientists, the early years in academia are demanding. To launch a successful research career, junior faculty must compete for research grants. But to win the grants, they need a track record of research accomplishments. While confronting this catch-22, they also must juggle heavy teaching loads.
Delany's award – intended specifically to support promising young investigators – meant a short respite from grant-writing and the time to read, among other things, the scientific literature on yeast telomeres.
Yeasty question
A telomere is a specialized DNA sequence, found at the end of chromosomes, that plays a critical role in cell replication, normal aging and diseases like cancer.
"I read the body of literature on yeast and wondered, 'What's going on with chickens and telomeres?' There was nothing," she said. "It was one of those 'aha' moments. I knew I'd hit it. I'd found a new area (of scientific inquiry) to move into."
In the decade since, Delany has come to dominate this area. She was the first to characterize the stability of telomeres and their regulation by telomerase, the enzyme responsible for keeping telomeres healthy, through the life cycle of the chicken – or indeed of any avian species.
Stopping the clock
In an article in the December 2000 issue of Development, Growth & Differentiation, she reported this work and her conclusion that the so-called telomere "clock" – which ensures cells don't live forever – functions in birds as well as mammals, and is disabled by cancer cells in both species as a way to evade mortality.
In 2003, her laboratory and one in Europe reported the characterization of two telomerase RNA genes found in the chicken herpesvirus, which is responsible for an avian cancer known as Marek's disease. And she was part of the international consortium of researchers that analyzed the draft sequence of the chicken genome, a landmark achievement published in the Dec. 9, 2004 issue of Nature. With her latest grant, Delany is investigating the role of telomeres in the pathology of Marek's disease in infected chickens.
The avian geneticist is confident the steadily accruing knowledge about the chicken genome will point the way to further advances that will benefit human health.
"We can't see now what those will be," she said. "But that's the nature of basic science."
The next leap forward may be an anti-telomerase vaccine to thwart cancer cells' efforts to sabotage telomere clocks. Or something no one has yet imagined – perhaps a development even more fantastic than using hens to fight cancer.




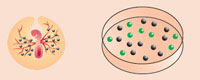
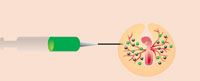 2. The transgenic cells are then injected into the circulatory system of new embryos, where they migrate to the developing gonads. The embryos are incubated until they hatch.
2. The transgenic cells are then injected into the circulatory system of new embryos, where they migrate to the developing gonads. The embryos are incubated until they hatch.