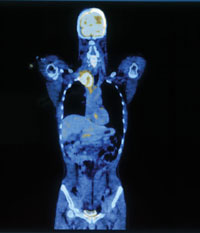
With PET-CT, doctors are able to see not just the size, shape and location of a tumor, but also how it behaves all — without lifting a scalpel
Since it first reached the market about four years ago, PET-CT scanning has become indispensable to oncology. Now UC Davis imaging scientists are pioneering new applications for the powerful technology.
"We're at the beginning of a very huge wave in molecular imaging that will revolutionize medicine," said David Shelton, professor and chief of nuclear medicine at UC Davis School of Medicine and Medical Center.
UC Davis acquired its first full-body PET-CT system in October 2004, the first cancer center in the Sacramento region to do so. Housed behind the medical center in a white trailer the size of a semi truck, the machine will soon be scanning 40 to 60 patients a week; most will be cancer patients.
Like an ordinary positron emission tomography, or PET, scan, PET-CT begins with an injection into the bloodstream of a short-lived radioactive glucose analog, F18-fluoro-deoxyglucose, or FDG. Over the course of 30 to 60 minutes, this radio-tagged sugar is taken up by the most metabolically active cells in the body, notably cancer cells. The
radiotracer then decays and disappears within several hours.
After receiving the FDG injection, the patient lies down on the narrow bed of the full-body PET-CT scanner. In 14 seconds, the computerized tomography, or CT, portion of the machine does its work, acquiring hundreds of images of the body, from head to mid-thigh. A computer then assembles the images into a three-dimensional, crisply detailed full-body CT picture.
The PET scanner takes its pictures next. Unlike the CT, which beams X-rays through the body, the PET scan reads signals emitted by the radio-tagged glucose that has been taken up
by the patient's cells.
Where the CT scan reveals fine anatomical detail, almost like a pen-and-ink medical illustration, the PET scan yields a fuzzier picture — more like an Impressionist painting — of a cellular function, in this case glucose metabolism. The combined PET and CT images reveal glucoseburning hotspots together with their precise anatomic location and size.
"PET-CT has changed the evaluation of oncology patients," said Gary Caputo, professor of radiology. "It can characterize lesions as to whether they are malignant or nonmalignant, stage a malignancy, and follow patients on therapy to characterize their response."
One recent patient came in for a PET-CT scan after surgery to remove a gastrointestinal stromal tumor. A follow-up CT had revealed areas of concern, suggesting some tumor might still remain.
But on PET-CT, the areas of concern all showed up "cold" — none was consuming glucose at a suspicious rate. Moreover, the areas remained "cold" a month later. The PET-CT study cleared the patient of disease and spared him an arduous course of chemotherapy.
Avoiding biopsy
PET-CT results can also help patients avoid biopsies. A suspicious finding on a lung X-ray, for example, can be ruled out as malignant using PET-CT, averting the need to take a tissue
sample.
In other cases, PET-CT results spare patients invasive surgeries, as when a scan reveals widespread metastatic disease, ruling out a surgical cure. "On a daily basis, cancer patients who come here for these scans have the management of their cancer changed as a result," said Rosalie Hagge, an assistant professor of radiology. "CT combined with PET is the most powerful way to image many kinds of cancer now."
But such applications are just the beginning. Shelton, who joined UC Davis in 1992, has assembled an imaging dream team that is breaking new technological ground and helping to define the future of diagnostic imaging.
He recruited Hagge from Duke University in 2002. Last year, he lured Caputo from UCSF and PET physicist Ramsey Badawi from Harvard University's Dana Farber Cancer Center.
The new faculty collaborate with biomedical engineer Simon Cherry, whose Davis lab is at the cutting edge internationally in molecular imaging research; radiochemist Julie Sutcliffe-Goulden, who has developed a rapid method of creating new molecular imaging probes; Kit Lam, an oncologist and combinatorial chemist who pioneered a rapid method of creating potential new anti-cancer compounds, and oncologists David Gandara, Derrick Lau and Primo "Lucky" Lara, who recently incorporated PET-CT into the first clinical trial at the Cancer
Center.
PET-CT in clinical trials
Typically, patients in a cancer clinical trial get a CT scan every three months to assess tumor response to an experimental therapy. In the first clinical trial at UC Davis Cancer Center to use PET-CT, investigators will perform the more powerful scans before therapy starts and again one week and six weeks afterward.
Tailored tracers
"We'll learn quicker and at less cost to patients," Badawi said. "We can use PET-CT to more rapidly predict success, and to change treatment faster in those who don't respond."
Preclinical research now under way at UC Davis includes efforts to identify new radioactive tracers that can join FDG in the molecular imaging toolkit. The goal is to create tracers that will be specific for a certain cancer — a tracer that will be taken up only by prostate cancer cells, for example, or only by squamous cell carcinomas.
Also under way are efforts to attach radioactive tracers to chemotherapy agents, so that a PET-CT scan can determine very early on whether a drug is reaching its target and doing its job UC Davis investigators are also creating new imaging hybrids, including a breast PET-CT and MR-PET fusion. With each step forward, the imaging scientists bring the future of oncology into clearer focus. It's looking brighter than ever.