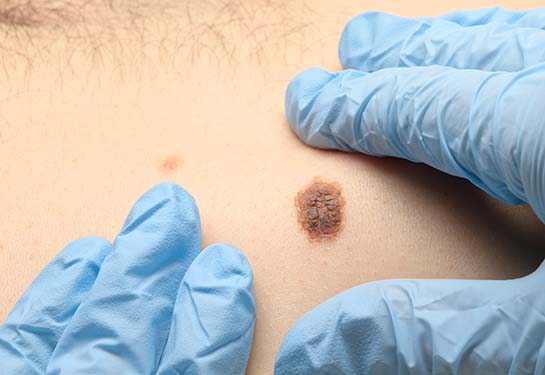
New gene profiling technology reveals melanoma biomarkers
UC Davis cancer researchers hope new technology will help diagnose and treat melanoma more successfully
A new UC Davis-led study sheds light on cell type-specific biomarkers, or signs, of melanoma. The research was recently published in the Journal of Investigative Dermatology.
Melanoma, the deadliest of the common skin cancers, is curable with early diagnosis and treatment. However, diagnosing melanoma clinically and under the microscope can be complicated by what are called melanocytic nevi—otherwise known as birth marks or moles that are non-cancerous. The development of melanoma is a multi-step process where “melanocytes,” or the cells in the skin that contain melanin, mutate and proliferate. Properly identifying melanoma at an early stage is critical for improved survival.
“The biomarkers of early melanoma evolution and their origin within the tumor and its microenvironment are a potential key to early diagnosis of melanoma,” said corresponding author of the study Maija Kiuru, associate professor of clinical dermatology and pathology at UC Davis Health. “To unravel the mystery, we used high-plex spatial RNA profiling to capture distinct gene expression patterns across cell types during melanoma development. This approach allows studying the expression of hundreds or thousands of genes without disrupting the native architecture of the tumor.”
The biomarkers of early melanoma evolution and their origin within the tumor and its microenvironment are a potential key to early diagnosis of melanoma.”—Maija Kiuru, UC Davis melanoma researcher
New technology used during study
The study examined the expression of over 1,000 genes in 134 regions of interest enriched for melanocytes, a cell in the skin and eyes that produces the pigment called melanin, as well as neighboring keratinocytes or immune cells. The tissue examined came from patient biopsies from 12 tumors, ranging from benign to malignant, using the NanoString GeoMx® Digital Spatial Profiler.
“We found that melanoma biomarkers are expressed by specific cell types, some by the tumor cells but others by neighboring cells in the so-called tumor microenvironment. The most striking observation was that S100A8, which is a known melanoma marker thought to be expressed by immune cells, was expressed by keratinocytes that make up the outermost layer of the skin called the epidermis,” said Kiuru. “Melanoma biomarkers in the epidermis have been largely overlooked in the past.”
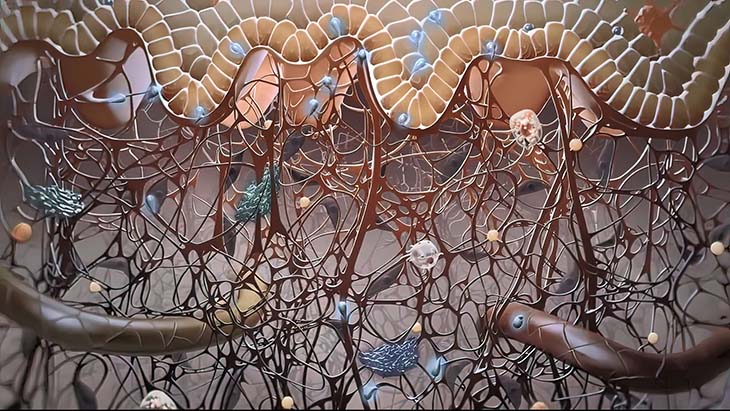
Keratinocytes are epidermal cells that have multiple functions, including forming a barrier against micro-organisms, heat, water loss, and ultraviolet radiation. Normal keratinocytes also control the growth of melanocytes.
“Unexpectantly, we discovered that S100A8 is expressed by keratinocytes within the tumor microenvironment during melanoma growth,” said Kiuru. “We further looked at S100A8 expression in 252 benign and malignant melanocytic tumors, which showed prominent keratinocyte-derived S100A8 expression in melanoma but not in benign tumors. This suggests that S100A8 expression in the epidermis may be a readily detectable indicator of melanoma development.”
Many molecular tests for diagnosis and prognosis of melanoma are gradually being introduced but markers of early melanoma development, particularly in the tumor microenvironment, remain lacking. In addition, although the treatment of metastatic melanoma has changed drastically since the development of immune checkpoint inhibitor therapies, biomarkers predicting the duration a patient will be cancer-free are largely unknown. Previous research has utilized sophisticated methods, including single-cell RNA sequencing, but has largely focused on melanoma metastases, or secondary tumor growths. This has overlooked the keratinocyte microenvironment of primary melanomas.
Other authors of the study include Michelle A. Kriner, Nanostring Technologies; Samantha Wong, UC Davis; Guannan Zhu, UC Davis and Xijing Hospital; Jessica R. Terrell, UC Davis; Qian Li, UC Davis; Margaret Hoang, Nanostring Technologies; Joseph Beechem, Nanostring Technologies; John D. McPherson, UC Davis.
Funding came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health (grant #K23AR074530) and Dermatology Foundation through Career Development Award in Dermatopathology.
UC Davis Comprehensive Cancer Center
UC Davis Comprehensive Cancer Center is the only National Cancer Institute-designated center serving the Central Valley and inland Northern California, a region of more than 6 million people. Its specialists provide compassionate, comprehensive care for more than 100,000 adults and children every year and access to more than 200 active clinical trials at any given time. Its innovative research program engages more than 240 scientists at UC Davis who work collaboratively to advance discovery of new tools to diagnose and treat cancer. Patients have access to leading-edge care, including immunotherapy and other targeted treatments. Its Office of Community Outreach and Engagement addresses disparities in cancer outcomes across diverse populations, and the cancer center provides comprehensive education and workforce development programs for the next generation of clinicians and scientists. For more information, visit cancer.ucdavis.edu.