UC Davis first in the state to offer life-changing therapy for Duchenne Muscular Dystrophy
Newly approved gene therapy treatment looks promising for halting progression of genetic disorder
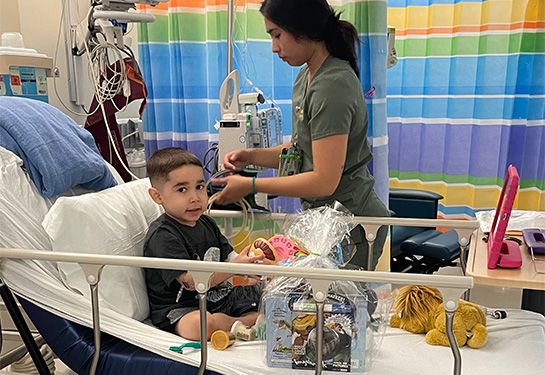
On August 4, the pediatric infusion room at UC Davis Medical Center was abuzz. A 5-year-old boy named Andrew Rodriguez was lying on a bed surrounded by family while receiving an infusion of the first-ever gene therapy for treating Duchenne muscular dystrophy (DMD) for pediatric patients.
"This is overwhelming! My heart is thumping! I'm kind of freaking out and happy he is getting this," exclaimed 8-year-old brother Caleb, standing nearby. "I can't believe he's the first kid in the state to be getting this!"
Indeed, Andrew made history that day as the first child in California to receive the groundbreaking, potentially lifesaving infusion treatment that was only recently approved by the U.S. Food and Drug Administration (FDA). He was the fourth child in the nation to receive the treatment outside clinical trials.

The treatment, years in the making, brings hope to as many as 12,000 people in the U.S., mostly males, who live with DMD, one of the most severe types of muscular dystrophy. DMD is a genetic disorder that leads to heart and breathing problems. Until the new treatment came along, families had little hope of a cure.
The disorder affects all muscles in the body, including those of the heart and lungs, causing them to weaken and atrophy gradually. The disease is caused by a change in a gene called dystrophin. This change leaves the body unable to make functional dystrophin protein, which generally acts like a shock absorber when muscles move. Without productive dystrophin protein, muscles weaken, stiffen, and become less flexible. As time passes, scars and fibrosis may further limit muscle function.
Most people start showing symptoms of the disorder as early as age 2. As the disease progresses, those with DMD lose their ability to walk and ultimately can’t breathe independently. Muscle deterioration often leads to premature death due to respiratory or cardiac failure, which typically occurs when patients are in their mid-20s or early 30s.
A disorder that Andrew inherited
Andrew’s saga began in April 2022, when he was 4. That’s when his pediatrician near the family’s home in Selma, south of Fresno noticed an enlargement in his calf muscles. Further evaluation confirmed what the doctor and parents already suspected: Andrew had DMD. His parents were no strangers to this genetic disorder. Andrew’s maternal uncle had also been diagnosed with DMD as a child and died from congestive cardiac failure at age 17.
Despite her brother’s death, when she learned about Andrew’s DMD, Jessica Rodriguez took a more hopeful attitude.
"I knew that Andrew's story would be different than my brother’s," Rodriguez said. "There were so many new medications and treatments from a decade ago. I knew we wouldn't go through what my family went through."
As it turns out, she was right. In 2018, Sarepta Therapeutics kicked off the first human clinical trials to evaluate the gene transfer therapy's safety and efficacy in boys with DMD.
I’ve been treating DMD patients for 30 years. To now have a situation where we can offer transformational, lifesaving gene therapy to these patients to replace a defective gene is incredibly gratifying and rewarding. It’s been an inspiring journey to be on.”—Craig McDonald
The search for a groundbreaking cure
Researchers have studied gene transfer therapy for years, but a breakthrough of this magnitude was unprecedented. By June 2023, the FDA had granted limited approval of gene therapy but only for children ages 4 and 5.

The treatment is a one-time infusion administered through a vein. The gene therapy works by replacing the Duchenne variant of dystrophin with a manufactured version of the dystrophin gene. The manufactured version allows the body to make a modified, shortened version of the dystrophin protein, thereby addressing the root cause of DMD.
The gene therapy makes a huge difference in slowing the progression of DMD.
"The typical patient of DMD will progressively develop difficulty getting up off the floor, difficulty sitting up on a chair, lose the ability to jump and eventually walking," explained Craig McDonald, professor and chair of the UC Davis Health Department of Physical Medicine and Rehabilitation. "The treatment seems to stabilize or halt the progression of the disease in follow-up observations. Kids who would normally be in wheelchairs by that point are jumping and running; they're practically indistinguishable from their non-DMD peers."
Andrew's parents learned about the success of clinical trials and recent FDA approval during a phone call this past June. There was only one problem, however. Treatment would have to start soon because Andrew was quickly nearing the cut-off age of 6-years-old set by the FDA. That's when his pediatric neurologist in Madera suggested the family connect with McDonald in Sacramento, a well-known DMD researcher. The health care teams worked quickly, and within a month Andrew was at UC Davis Medical Center to receive the infusion.
It happened just in time, three days before his sixth birthday.
Andrew's dad, Placido Rodriguez, credits the tireless work of UC Davis Health, Sarepta Therapeutics, California Children's Services, Valley Children's Healthcare, and the selfless individuals who understood that time was of the essence and the infusion was possible.
"It was a big collaboration on everyone's part. I can't get over it; it was amazing to see God work through people, it was bigger than just Andrew’s gene therapy," Placido said. "They fought for us. We are so humbled.”
Standing beside her son on the day of infusion, Jessica Rodriguez teared up as she described her feelings upon seeing Andrew receive the treatment.
"Last year, I said, 'God, whatever happens, I pray we see your grace through everyone we meet,' and just a year later, we find out this is happening," Rodriguez said. "We're still in shock. I think, who are we to be here, just a year later, knowing his story will be different than other little boys’.”
Being part of this historic moment is a career highlight for McDonald, an award-winning researcher in the field of neuromuscular disorders.
"I've been treating DMD patients for 30 years," McDonald said. "To now have a situation where we can offer transformational, life-saving gene therapy to these patients to replace a defective gene is incredibly gratifying and rewarding. It's been an inspiring journey to be on."