Building on basics: UC Davis achieves the highest honor in oncology
 From the beginning,
From the beginning,
de Vere White proclaimed that only a collective effort – not just within the center but embracing outside partners as well – could convert UC Davis from a respected cancer treatment center to a multi-faceted research and clinical powerhouse.
"We were young, we were small, and although we certainly had a lot of talent and potential, we hadn’t really made a mark," Gandara recalls.
Just 20 years later, UC Davis has surged from semi-obscurity to the big leagues in cancer treatment and research, winning designation in February as a "comprehensive" cancer center by the National Cancer Institute (NCI).
By earning comprehensive status, UC Davis becomes only the 41st cancer center validated by the NCI as providing first-rate cancer care combined with a strong research base and wide spectrum of cancer prevention, education and outreach services.
"It’s amazing," says Gandara, a leading lung cancer specialist. "To come so far so fast and to now be numbered among the most prestigious cancer centers in the country is simply extraordinary."
While many cancer treatment facilities include the word "comprehensive" in their names, the term as used by the NCI is very specific, conveying a special state of excellence awarded only after a cancer center passes a rigorous review.
"This is not a beauty contest," explains Ralph de Vere White, the center’s long-time director. "It’s a special seal of approval, and it means our community can count on the fact that we’re good – good in cancer education, cancer care, cancer research, and all other aspects of cancer.
"For patients in the valley, the bottom line is that they do not have to get on a plane and fly somewhere else for world-class cancer treatment."
 Making headlines
Making headlines
"Cancer center joins elite level"
"‘Comprehensive’ status brings name change to cancer center"
"New cancer center label boosts UCD"
There were no secrets or shortcuts underlying UC Davis’ climb to the top. Rather, success was a product of hard work, vision and passion shared by a broad swath of clinicians, researchers and cancer center staff soldiering for two decades toward a common goal.
Convinced that UC Davis had the raw material to break into the upper echelon of cancer centers, this team followed a carefully plotted path, including recruitment of the best and brightest scientists and doctors; construction of a 56,000-square-foot treatment center and state-of-the-art companion research laboratory; diversification of the center’s cancer programs; expansion of its clinical trials; and the formation of fruitful connections with distinctive partners, from the schools of veterinary medicine and agriculture to the California Cancer Registry and the Lawrence Livermore National Laboratory.
Also key was inspired leadership at the top, namely, the force of nature who has led the cancer center since his appointment as director in 1996, de Vere White. A urologic oncologist by training, de Vere White remains one of the top genitourinary cancer surgeons in the country, beloved by patients who consistently rate him one of "The Best Doctors in America."
From the beginning, de Vere White proclaimed that only a collective effort – not just within the center but embracing outside partners as well – could convert UC Davis from a respected cancer treatment center to a multi-faceted research and clinical powerhouse. As he puts it, "You can be the coach, but you can’t be the quarterback, the running back and all the rest of it. So I simply try to put good people together, and we go toward our goal as a team."
A steady ascent to the top
The first goal was achieved in 2002, when UC Davis earned designation by the NCI, becoming the 61st such cancer center in the country. The NCI, the nation’s top cancer organization, awards designation only to cancer institutions with the demonstrated potential to make major scientific contributions to the war against cancer. Granted after an exhaustive review process, NCI designation brings multiple benefits, from a glow of prestige that aids recruitment to a stable stream of federal research dollars.
Designation was renewed for UC Davis in 2006. But de Vere White and his allies were not content to stop there. All along, they had their eyes on a loftier honor – comprehensive status.
The journey to that next milestone has not been an easy one. Compared to cancer centers in larger metropolitan areas, UC Davis has a smaller population base, which means fewer patients to participate in clinical trials of promising cancer drugs and a tougher climate for fundraising, a component ever more critical for scientists as government research support has dwindled.
But UC Davis also has access to some rare assets. For one thing, it is cocooned within the hold of a powerful mother ship – one of the most accomplished, dynamic university systems in the world, an institution offering untold opportunities for collaboration. In addition, Sacramento is one of the most racially diverse cities in the nation, a territory begging for cancer research related to ethnic disparities.
Why not, de Vere White and others reasoned, develop and strengthen the cancer center through integration of these special components? Wouldn’t a united front spanning multiple disciplines and partners serve as an even more potent weapon against cancer?
"Cancer is a very complicated disease, and it takes commitment from all elements of the university, the health system and beyond to attack it from every angle," says Karen Kelly, associate director for clinical research. "I think UC Davis really understands that, and I think our record reflects it."
Harnessing technology
 "We are developing technologies for use on so many levels. The challenge of the next 10 to 20 years is to get these ideas from the lab to the clinic and, ultimately, to commercialization."
"We are developing technologies for use on so many levels. The challenge of the next 10 to 20 years is to get these ideas from the lab to the clinic and, ultimately, to commercialization."
A good number of the cancer center’s team works within one of its most unique partner components, the Department of Biomedical Engineering, on the main UC Davis campus. Led by physicist Simon Cherry, the Biomedical Technology program is one of the cancer center’s four core pillars, teaming physical scientists and engineers with medical experts to develop tools for cancer detection, treatment and monitoring.
Formerly anchored in work at Lawrence Livermore National Laboratory, the program’s scope has expanded well beyond the national lab in recent years, and now includes more than 30 members. The program has been lauded by peers for being highly innovative and productive, and is considered a unique national resource.
A stroll through the warren of laboratories at biomedical engineering headquarters, which overlooks the Aggies football stadium, illuminates the promise of technology as a weapon against cancer. Off one hallway, Laura Marcu and a team of graduate students are refining a hand-held, fiberoptic probe that helps surgeons distinguish malignant tissue from healthy tissue during surgery to remove brain, head and neck tumors. The probe, which already has been used on patients, uses fluorescent light to obtain a molecular signal analyzed in real time to determine whether tissue is healthy or cancerous. Next step: broader clinical trials to determine parameters for most effective use.
In another laboratory, Katherine Ferrara, founding chair of the Department of Biomedical Engineering, is hunting for ways to target delivery of cancer drugs to tumors in ways that improve efficacy and reduce toxic side effects. One avenue involves encapsulating the drug in nanoparticles and then using ultrasound to help guide delivery of the drug and enhance its release at the target.
"We are developing technologies for use on so many levels," says Cherry, whose own work focuses on developing new molecular imaging technologies to better identify cancerous cells and help develop and validate new drugs. "The challenge of the next 10 to 20 years is to get these ideas from the lab to the clinic and, ultimately, to commercialization."
A little help from our best friends
 Uniting veterinary scientists with oncologists, the program aims to extend the lives of small animals with cancer through treatments and techniques that could benefit human patients, as well.
Uniting veterinary scientists with oncologists, the program aims to extend the lives of small animals with cancer through treatments and techniques that could benefit human patients, as well.
While Cherry’s team uses technology to fight cancer, the School of Veterinary Medicine lends its know-how and its animal patients to the cause through another of UC Davis’ partnerships, its comparative oncology program. Few cancer centers can boast an alliance with a world-renowned veterinary school right in its front yard, and UC Davis is making full use of the connection. Uniting veterinary scientists with oncologists, the program aims to extend the lives of small animals with cancer through treatments and techniques that could benefit human patients, as well.
The UC Davis Veterinary Teaching Hospital sees more than 1,300 animals, primarily dogs, with spontaneous cancers each year, a population that doubles as a resource for human cancer researchers. Compared to mice, dogs make excellent models for human research because of their genetic diversity, exposure to the same environments as people, and because they get some of the same cancers.
Clinical trials of anti-cancer drugs in pets are also less costly than in humans, because charges for everything from blood tests to CT scans are lower in veterinary medicine. A trial that might take five years and millions of dollars with human volunteers can be completed in five months at far less expense with animals.
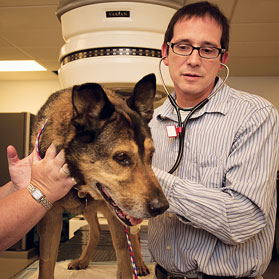 Few cancer centers can boast an alliance with a world-renowned veterinary school right in its front yard, and UC Davis is making full use of the connection.
Few cancer centers can boast an alliance with a world-renowned veterinary school right in its front yard, and UC Davis is making full use of the connection.
"Because disease can progress quicker in dogs and because their lifespan is much shorter than ours, it is easy to follow a dog through its life and track the effectiveness of certain cancer drugs," says Michael Kent, an associate professor in the School of Veterinary Medicine. "This whole area of using animal models as a bridge to fight cancer is really game-changing, and I believe it will take us to a new level in cancer research."
Among the notable work already accomplished is an initial clinical trial Kent conducted with help from Kit Lam, professor and chair of the Department of Biochemistry and Molecular Medicine and an expert in targeted drug therapies for cancer. The trial, involving dogs with lymphoma, used Lam’s nanoparticle technology to deliver the drug paclitaxel, used to treat lung, ovarian and breast cancer in humans.
While the principal goal was to develop a formulation of the drug that dogs could tolerate, the study and planned subsequent trials could yield results beneficial to human patients who need chemotherapy.
Tackling disparities in cancer outcomes
In addition to caring for individual patients, NCI-designated cancer centers are charged with improving the health of communities, and in its march toward comprehensive status, UC Davis has embraced that mission with gusto.
 In addition to caring for individual patients, NCI-designated cancer centers are charged with improving the health of communities, and in its march toward comprehensive status,
In addition to caring for individual patients, NCI-designated cancer centers are charged with improving the health of communities, and in its march toward comprehensive status,
UC Davis has embraced that mission with gusto.
Under Moon Chen, a professor of hematology and oncology, the cancer center’s Population Sciences & Health Disparities Program is a national leader in closing gaps in cancer outcomes for different populations. With 24 members from seven departments, the program boasts peer-reviewed grants funding studies on every racial/ethnic group except Hawaiians and Pacific Islanders.
One standout is Chen’s leadership of an NCI-funded national effort to curb cancer among Asian Americans, who are disproportionately affected by the disease. Headquartered at UC Davis, the Asian American Network for Cancer Awareness, Research and Training blends community-based education with training and research. For the last five years, Chen and colleagues at UCLA and UCSF joined forces on a project exploring how best to persuade Asian Americans to undergo testing for hepatitis B, a leading risk factor for liver cancer.
Another innovative outreach program sought to increase the rate of mammography screening for American-Indian and Alaska-native women, who have disproportionately high death rates from breast cancer. Marlene von Friederichs-Fitzwater, director of the Outreach Research and Education Program, found that cultural beliefs are key barriers to breast cancer screening among native women. She worked with members from eight tribes to develop a DVD using traditional storytelling and talking circles to communicate the importance of mammography.
 "We can all congratulate ourselves for the moment, but then we will be expected to deliver. And we will."
"We can all congratulate ourselves for the moment, but then we will be expected to deliver. And we will."
Results of the "Mothers’ Wisdom Breast Health Program" showed that of 118 women who watched the DVD and said they would get a mammogram, nearly all of them (112) had done so within the following year. The program has since expanded to 25 Northern California tribes.
Meanwhile, family physician Tony Jerant won an NCI grant to investigate low colorectal screening rates among Latinos and African-Americans, who have disproportionately high rates of colon cancer. Moving beyond traditional outreach methods, Jerant developed an interactive program that educates patients in doctors’ waiting rooms, alerting them to the importance of screening. Promising early results led to supplemental funding that expanded the program to four other cities.
With 3.4 million cancer cases, representing 95 percent of all cases in the state, the registry provides a wealth of opportunities for collaboration and is the world’s largest, most ethnically diverse registry in a geographically contiguous area.
Bolstering these and other health disparities work is another of UC Davis’ unique resources – the California Cancer Registry. With 3.4 million cancer cases, representing 95 percent of all cases in the state, the registry provides a wealth of opportunities for collaboration and is the world’s largest, most ethnically diverse registry in a geographically contiguous area.
"The registry is truly a treasure," Chen says. "It provides us with an enormous, tremendously diverse data base that we can use to answer any imaginable question related to cancer."
Putting patients first
 At the heart of all the cancer center’s work, of course, is the health of the patient – specifically, what can be done to make sure more people beat cancer and experience fewer side effects along the way.
At the heart of all the cancer center’s work, of course, is the health of the patient – specifically, what can be done to make sure more people beat cancer and experience fewer side effects along the way.
At the heart of all the cancer center’s work, of course, is the health of the patient – specifically, what can be done to make sure more people beat cancer and experience fewer side effects along the way. Toward that end, the testing and development of new drugs through clinical trials is a key priority – and a fourth area of excellence that propelled UC Davis toward comprehensive status.
In 2002, UC Davis received approximately $42 million in federal research funding through a multitude of grants. By 2011, that had grown close to $108 million. In addition, the number of National Institutes of Health research project (R01) grants more than doubled, from 50 to 126.
With such support, coupled with fundraising, UC Davis has developed a robust program of investigator-initiated clinical trials, with 24 such studies under way, testing a wide variety of agents. And that, says Karen Kelly, the cancer center’s Phase 1 clinical director, is only the tip of the iceberg.
"There are thousands of great ideas out there – about promising therapies, tools to prevent cancer, how to help survivors – but all of these require clinical trial testing," Kelly says. "Now, with comprehensive status, we’ll be in a better position to take these ideas from the laboratory to the bedside, and translate what we do into help for patients."
That, says de Vere White, is the overriding goal shared throughout the UC Davis Comprehensive Cancer Center – and a challenge that will dominate the center’s agenda for the next five years.
"We are not going to fly under the radar any longer, and we are not going to be looked at as the surprise that did more with less," says de Vere White. "We can all congratulate ourselves for the moment, but then we will be expected to deliver. And we will."
















