CPMDS Highlights

2026
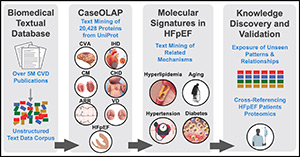
Natural language processing of biomedical text to map and prioritize protein–disease associations in HFpEF
March 2026
Abstract
The validation of promising clinical biomarkers, molecular mechanisms, and novel drug targets in cardiovascular disease (CVD) is hindered by a vast and fragmented biomedical literature, which now exceeds 38 million publications indexed in PubMed. To address the central challenge of navigating and synthesizing a huge fragmented biomedical literature base, we applied our validated machine learning–based text-mining algorithm containing natural language processing (NLP) and incorporated this into a ValIdated Text-mining using Advanced Language model (VITAL) as a complementary framework.
2nd Worldwide Sodium Channel Conference 2026

February 2026 — Vladimir Yarov-Yarovoy attended the 2nd World Sodium Channel Conference in Grindelwald, Switzerland, and presented a recently published research project entitled “Exploring voltage-gated sodium channel conformations and protein–protein interactions using AlphaFold2”. This study demonstrates the power of deep learning–based structural methods to advance understanding of voltage-gated sodium channel gating and drug modulation, while emphasizing that computationally predicted states remain hypotheses requiring experimental validation.
Theranostics Digital Twins: A Path to Personalized Medicine

January 2026 — On January 14, the Data Science Seminar Series has tackled an intersection of two critical data science concepts in cancer research: theranostics and digital twins. Emilie Roncali, an associate profession at UC Davis, gave an overview of how these concepts work in practice in liver, prostate, and metastatic cancer.
GenScript Life Science Research Grant (LSRG) for AI Drug Discover Project

January 2026 — Vladimir Yarov-Yarovoy received the GenScript Life Science Research Grant (LSRG) for AI Drug Discover project. Yarov-Yarovoy’s project focuses on targeting voltage-gated ion channels, which are pivotal in regulating electrical activity in excitable cells and are critical pharmaceutical targets for treating many diseases, including cardiac arrhythmias and neuropathic pain. The LSRG Committee has evaluated over five hundred applications during this review cycle. And selected 19 projects for funding.
Towards credible digital twins for basic and preclinical research

January 2026
Abstract
Digital twins are well established in industrial settings, but there has not been wide adoption in biomedical settings. Digital twins for biomedical applications are now possible with the inclusion of artificial intelligence and the potential to combine mechanistic and clinical models that learn and adjust for human variability.
Digital Twins as New Approach Methodologies (NAMs) in Biophotonics

January 2026 — Led by Moderator Colleen Clancy, Director of the Center for Precision Medicine at Univ. of California, Davis, panelists will explore the transformative role of digital twin technology in personalized medicine, treatment optimization, and medical device simulation. Discussions will include the coupling of AI-driven insights in patient care through digital twins, emerging applications of digital twins, and how this revolutionizing technology has the potential to drive the healthcare protocols of the future.
2025
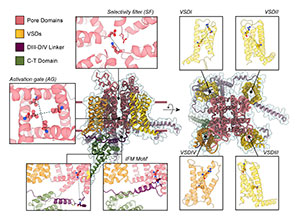
Mapping the Conformational Landscape of Human Voltage-Gated Sodium Channels with AlphaFold
December 2025
Abstract
Voltage-gated sodium (NaV) channels are central players in electrical signaling across biological systems, shaping excitability, information processing, and physiological function in health and disease; understanding their molecular, structural, and energetic mechanisms is therefore essential for deciphering channel dysfunction and enabling the development of next-generation, more precise and personalized therapeutic strategies. Researchers at UC Davis’s Center for Precision Medicine and Data Sciences report a new AlphaFold2-based computational modeling framework for moving beyond single “snapshot” structures toward a more dynamic view of the conformational landscape that underlies NaV channel gating, regulation, and pharmacology. By enhancing conformational sampling using subsampled multiple-sequence alignments and varying the number of recycles, the team generated ensembles of full-length models across the human NaV1.1–NaV1.9 family that recapitulate experimentally observed conformations, propose additional states not yet described experimentally, and suggest potential intermediate conformations. Correlation and clustering analyses then organize these large ensembles into recurrent, interpretable state groups and reveal coordinated behavior across key functional regions. In parallel, the study benchmarks AlphaFold Multimer for NaV protein–protein interactions, showing high-accuracy models of complexes with auxiliary β subunits and calmodulin, and demonstrating that these partners can reshape the modeled conformational landscape and coupling between functional states. Together, the work provides a reproducible, hypothesis-generating workflow to model and analyze NaV structural ensembles, providing a foundational step toward capturing the full complexity of NaV channel dynamics and modulation, a key requirement for developing next-generation, structure-guided, and ultimately more personalized therapeutic strategies.
From Fire to Ice: The Future of Thermal Precision Medicine
By Colleen E. Clancy, Editor in Chief, Journal of Precision Medicine: Health and Disease

December 2025 — A woman walks into the forest on a winter morning. The air bites at her skin as she wades into a pool carved from glacial runoff. Her breath catches, her vessels constrict, and within seconds, her physiology responds and reconfigures to ensure short term survival. Was this scene set a millennium ago in Norway, or today in the California Sierra Nevada mountains?
Humans have long utilized temperature as therapy. The Edwin Smith Papyrus of ancient Egypt (ca. 3500 BCE) describes cooling inflamed wounds. Hippocrates prescribed cold-water immersion for vitality, while Roman bathhouses included the frigidarium for restoration. In Finland, avantouinti, or winter swimming, is both ritual and medicine. Across centuries, the principle endures: the controlled use of heat and cold to promote health, healing and resilience. It should not be forgotten that the history of thermal research also carries a sinister legacy: during World War II, Nazi physicians conducted the Dachau hypothermia experiments, in which prisoners were subjected to lethal cold in the name of “science”. The pursuit of physiological knowledge must always be bound by ethics and humanity.
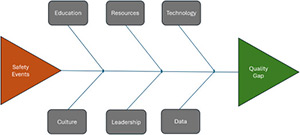
Health Care Quality and Patient Safety in the Era of Artificial Intelligence
December 2025
Abstract
The integration of digital health technologies is revolutionizing health care quality and patient safety by shifting from reactive to proactive care models. Advances in electronic health records, clinical decision support systems, data analytics, artificial intelligence (AI), mobile applications, and tele-health are enhancing clinical decision-making, patient engagement, and care coordination. While these technologies offer significant benefits, challenges related to data privacy, integration into workflows, and health disparities require ongoing attention to ensure that all patients can benefit from these innovations. Adoption of digital health and AI to bring systemness and real time information delivery can support delivery of high-quality care.
The Physiological Society’s Member Forum

December 2025 — Publishing in the Physiological Society’s family journals supports the future of physiology. To tell us more about research and publishing, the journal's Editors-in-Chief, including Director Colleen Clancy, took to the stage for the In Conversation session at the Member Forum.
Digital Twins and Precision Medicine for Cardiovascular Disease
 Colleen Clancy, Ph.D. — Institute for Precision Medicine, UC Davis
Colleen Clancy, Ph.D. — Institute for Precision Medicine, UC Davis
UC Davis Heart Research Day 2025 Keynote Presentation

October 2025 — Director Colleen Clancy presented groundbreaking work from the Center on the development and application of digital twin technologies to revolutionize cardiovascular care. Her talk highlighted how computational modeling, artificial intelligence, and multimodal biomedical data integration are converging to create individualized virtual replicas or “digital twins” of the human heart.
Digital twins enable researchers and clinicians to simulate disease progression, test therapeutic interventions, and predict patient-specific outcomes with unprecedented precision. Clancy discussed current advances in modeling cardiac dysfunction and recovery, and showcased how this approach is laying the foundation for next-generation diagnostics and personalized therapies in cardiovascular medicine.
CPMDS Featured as Transforming Biomedical Discovery through HPC

October 2025 — The Center for Precision Medicine and Data Sciences team uses HPC@UCD to study biomedical challenges across multiple scales by integrating molecular modeling, large-scale data analytics, and machine learning. Their work spans from building and simulating large protein systems, such as voltage-gated ion channels, studying how modulators influence their structure and function; analyzing large-scale omics datasets to identify disease-related genes, proteins, and networks; developing predictive AI models that capture the effects of genetic variants; and simulating populations of synthetic cardiomyocytes to model electrophysiological variability and drug sensitivity.
Director Clancy served as Co-Chair and Plenary Speaker at NIH Digital Twins Workshop

September 2025 — Director Colleen E. Clancy, Ph.D., recently co-chaired the NIH Digital Twins in Heart, Lung, Blood, and Sleep Research Workshop, held virtually on September 25–26, 2025. This two-day meeting brought together leaders across science, medicine, and engineering to advance the role of Digital Twins in precision health. More information and video casts can be found here.
In addition to co-chairing the workshop, Clancy delivered the Plenary Lecture and presented a second talk on the development of precision digital twins in cardiovascular and translational medicine.

The NIH has embraced the concept of Digital Twins, as outlined in a 2024 report from the National Academies of Science, Engineering, and Medicine (NASEM):
“A Digital Twin is a set of virtual information constructs that mimics the structure, context, and behavior of a natural, engineered, or social system (or system-of-systems), is dynamically updated with data from its physical twin, has a predictive capability, and informs decisions that realize value. The bidirectional interaction between the virtual and the physical is central to the Digital Twin.”
This workshop followed the NASEM framework while highlighting applications in Heart, Lung, Blood, and Sleep (HLBS) research. The goals were to showcase the latest scientific advances, identify research gaps, and catalyze collaborations. Sessions also emphasized how NIH resources—including Biodata Catalyst and NHLBI-AI—can accelerate Digital Twin development.
By convening clinicians, scientists, engineers, entrepreneurs, and program staff, this workshop marked an important step in building a robust community of practice around Digital Twins in HLBS research.
Director Clancy presented at the Bay Area Cardiovascular Research Symposium at Stanford University in August 2025
August 2025 — Director Clancy presented at the Bay Area Cardiovascular Research Symposium, “Digital Twins: From Molecule to Medicine”. Stanford University. August 2025. She discussed how digital twins can be applied across multiple disciplinary domains and application from drug discover to digital twin guided clinical trials.
Khoa Ngo Receives UC Davis Business Development Fellowship

August 2025 — Khoa Ngo, Ph.D., a Postdoctoral Fellow in the Center for Precision Medicine and Data Science at UC Davis, was awarded a Business Development Fellowship from the UC Davis Mike and Renée Child Institute for Innovation and Entrepreneurship. The fellowship helps researchers learn how to bring new ideas from academic research into real-world settings, whether through industry partnerships or new ventures. Through MBA-level coursework, interdisciplinary practicums with MBA students, and guidance from faculty, investors, and entrepreneurs, Ngo is gaining practical training in evaluating the potential of emerging technologies and communicating their value to diverse stakeholders, strengthening the translational reach of his work in computational precision medicine.
Colleen Clancy and Vladimir Yarov-Yarovoy appointed to HESI Conduction in Silico Subteam

June 2025 — In June 2025, Colleen Clancy attended the Biannual Meeting of the National Academy of Sciences in Washington, D.C. She and Yarov-Yarovoy were recently appointed to the the Health and Environmental Sciences Institute (HESI) Conduction In Silico Subteam working group. These appointments underscore the Center leadership in computational modeling of cardiac electrophysiology and her commitment to advancing regulatory science. As subteam lead and member, they will guide efforts to develop and refine in silico tools for evaluating conduction-related cardiac safety liabilities, contributing to cross-sector collaboration at the interface of pharmacology, toxicology, and computational biophysics.
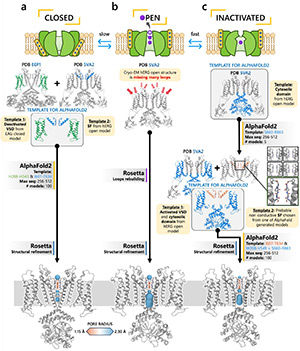
Harnessing AlphaFold to reveal hERG channel conformational state secrets
June 2025
Abstract
 To design safe, selective, and effective new therapies, there must be a deep understanding of the structure and function of the drug target. One of the most difficult problems to solve has been resolution of discrete conformational states of transmembrane ion channel proteins. An example is Kv11.1 (hERG), comprising the primary cardiac repolarizing current, Ikr. hERG is a notorious drug anti-target against which all promising drugs are screened to determine potential for arrhythmia. Drug interactions with the hERG inactivated state are linked to elevated arrhythmia risk, and drugs may become trapped during channel closure. However, the structural details of multiple conformational states have remained elusive. Here, we guided AlphaFold2 to predict plausible hERG inactivated and closed conformations, obtaining results consistent with multiple available experimental data. Drug docking simulations demonstrated hERG state-specific drug interactions in good agreement with experimental results, revealing that most drugs bind more effectively in the inactivated state and are trapped in the closed state. Molecular dynamics simulations demonstrated ion conduction for an open but not AlphaFold2 predicted inactivated state that aligned with earlier studies. Finally, we identified key molecular determinants of state transitions by analyzing interaction networks across closed, open, and inactivated states in agreement with earlier mutagenesis studies. Here, we demonstrate a readily generalizable application of AlphaFold2 as an effective and robust method to predict discrete protein conformations, reconcile seemingly disparate data and identify novel linkages from structure to function.
To design safe, selective, and effective new therapies, there must be a deep understanding of the structure and function of the drug target. One of the most difficult problems to solve has been resolution of discrete conformational states of transmembrane ion channel proteins. An example is Kv11.1 (hERG), comprising the primary cardiac repolarizing current, Ikr. hERG is a notorious drug anti-target against which all promising drugs are screened to determine potential for arrhythmia. Drug interactions with the hERG inactivated state are linked to elevated arrhythmia risk, and drugs may become trapped during channel closure. However, the structural details of multiple conformational states have remained elusive. Here, we guided AlphaFold2 to predict plausible hERG inactivated and closed conformations, obtaining results consistent with multiple available experimental data. Drug docking simulations demonstrated hERG state-specific drug interactions in good agreement with experimental results, revealing that most drugs bind more effectively in the inactivated state and are trapped in the closed state. Molecular dynamics simulations demonstrated ion conduction for an open but not AlphaFold2 predicted inactivated state that aligned with earlier studies. Finally, we identified key molecular determinants of state transitions by analyzing interaction networks across closed, open, and inactivated states in agreement with earlier mutagenesis studies. Here, we demonstrate a readily generalizable application of AlphaFold2 as an effective and robust method to predict discrete protein conformations, reconcile seemingly disparate data and identify novel linkages from structure to function.
Colleen Clancy and Igor Vorobyov Present at HRS April, 2025


April 2025 — At the 2025 Heart Rhythm Society Scientific Sessions, Colleen Clancy and Igor Vorobyov delivered cutting-edge presentations highlighting advancements in cardiac electrophysiology modeling. Clancy discussed the integration of multiscale digital twin technologies for predictive simulation of arrhythmogenic risk, while Vorobyov showcased novel computational frameworks for ion channel dynamics and membrane interactions. Together, their work emphasized the power of interdisciplinary modeling in driving precision medicine for heart rhythm disorders.
Clancy and Yarov-Yarovoy Present at the April 2025 British Pharmacological Society Meeting

April 2025 — At the 2025 British Pharmacological Society Meeting, Colleen Clancy and Vladimir Yarov-Yarovoy presented their collaborative work in a session entitled “Toward Design of Biologics Targeting Ion Channels.” Their talk highlighted recent advances in computational modeling and rational design strategies aimed at developing next-generation peptide therapeutics that selectively modulate ion channel function. Clancy shared insights into multiscale simulations and digital twin approaches for evaluating drug-channel interactions in silico, while Yarov-Yarovoy focused on structure-based design and optimization of biologics derived from natural toxins. Together, their work represents a major step forward in precision pharmacology targeting excitable tissues.
The Journal of Precision Medicine: Health and Disease

March 2025 — So excited to launch this new journal from the Physiological Society as a venue for precision medicine. We offer free open access publication for the first two years! Reach out to discuss a submission, or take a look at our guide to authors - we likely have an article type to suit your needs. Have a research breakthrough that can be described in one figure? We can guarantee rapid turnaround - and did I mention free open access?
Watch the full video of the conversation with Editor-in-Chief, Colleen Clancy, who introduces the Journal of Precision Medicine: Health and Disease, one of the newest additions to the Physiological Society's family of journals.
Presentations by Clancy and Hernandez-Hernandez at the February 2025 Biophysical Society National Meeting

February 2025 — At the 2025 Biophysical Society National Meeting, Colleen Clancy and Gonzalo Hernandez-Hernandez delivered insightful presentations showcasing the intersection of biophysics and computational modeling. Clancy explored state-of-the-art digital twin methodologies applied to cardiac electrophysiology, emphasizing predictive modeling approaches for drug safety and arrhythmia susceptibility. Hernandez-Hernandez presented, "A Computational Model of Angiotensin II Receptor Type 1 Dynamics: Insights into Vascular Regulation". Their contributions underscored the critical role of biophysical modeling in advancing translational research and precision medicine.
Director Clancy co-authored the Cambridge based Public Health Genomics Foundation report to the British Parliament entitled “Physiology Passport: Putting Personalized Prevention at the Heart of Resilient Health Systems” steering group

January 2025 — Professor Colleen Clancy has contributed to the “Physiology Passport: Putting Personalized Prevention at the Heart of Resilient Health Systems” steering group. A copy of the report can be found at: physoc.org/PhysiologyPassport.
The project has been a fantastic opportunity to bring Clancy's expertise in the area to understand how physiological research, in concert with genetics, epidemiology, behavioral science and other disciplines, can support a personalized approach to preventative health.
Building digital twins and hearts
January 2025 — Imagine having a digital carbon copy of yourself that physicians could use to predict long-term risks for disease, assess how your body may respond to treatment, and simulate surgeries in advance. A virtual twin may sound as far-fetched as robotic surgery and self-driving cars once did, but researchers are studying how to turn this vision into a reality.
“I hope I see it in my lifetime,” said Colleen E. Clancy, Ph.D., the director of precision medicine and data sciences at the University of California, Davis Health and School of Medicine. “I think we’re on the cusp of real, personalized medicine.”
2024
An overview of drug-induced sodium channel blockade and changes in cardiac conduction: Implications for drug safety

December 2024
Abstract
The human voltage-gated sodium channel Nav1.5 (hNav1.5/SCN5A) plays a critical role in the initiation and propagation of action potentials in cardiac myocytes, and its modulation by various drugs has significant implications for cardiac safety. Drug-dependent block of Nav1.5 current (INa) can lead to significant alterations in cardiac electrophysiology, potentially resulting in conduction slowing and an increased risk of proarrhythmic events. This review aims to provide a comprehensive overview of the mechanisms by which various pharmacological agents interact with Nav1.5, focusing on the molecular determinants of drug binding and the resultant electrophysiological effects. We discuss the structural features of Nav1.5 that influence drug affinity and specificity. Special attention is given to the concept of state-dependent block, where drug binding is influenced by the conformational state of the channel, and its relevance to therapeutic efficacy and safety. The review also examines the clinical implications of INa block, highlighting case studies of drugs that have been associated with adverse cardiac events, and how the Vaughan-Williams Classification system has been employed to qualify “unsafe” sodium channel block. Furthermore, we explore the methodologies currently used to assess INa block in nonclinical and clinical settings, with the hope of providing a weight of evidence approach including in silico modeling, in vitro electrophysiological assays and in vivo cardiac safety studies for mitigating proarrhythmic risk early in drug discovery. This review underscores the importance of understanding Nav1.5 pharmacology in the context of drug development and cardiac risk assessment.
Colleen Clancy Presented at the Seventh Annual Reimagine Health Research Symposium

December 2024 — Colleen Clancy, Ph.D., professor and director of Precision Medicine and Data Sciences, was an invited presenter at the Seventh Annual Reimagine Health Research Symposium hosted at The University of Arizona College of Medicine – Phoenix Campus in downtown Phoenix, Arizona and virtually via Zoom. Her presentation title was, "Digital twins from the atom to the neighbor".
The 7th Annual reimagine Health Research Symposium on Artificial Intelligence in Medical Practice and Research concerns the emerging and rapidly growing use of AI and machine learning in medicine, with a focus on how AI can improve medical practice, in terms of diagnoses and medical forecasting, as well as applications, and on how AI can be used to accelerate medical research. It will also be discussed how this new technology will benefit both practitioners and patients and how it will better inform rapid acceleration of research progress, as it has in other fields.
Society welcomes inaugural Editors-in-Chief for The Journal of Precision Medicine: Health and Disease and The Journal of Nutritional Physiology

December 2024 — Following the announcement of The Physiological Society’s partnership with Elsevier to launch a new journal, we are delighted to introduce the Editor-in-Chief (Colleen E. Clancy) and Deputy Editor in Chief (Vladimir Yarov-Yarovoy) of The Journal of Precision Medicine: Health and Disease.
Mechanisms of Chemical Atrial Defibrillation by Flecainide and Ibutilide
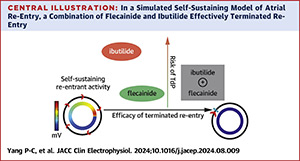
December 2024
Abstract
Pharmacological approaches to atrial defibrillation offer noninvasive, low-risk, and cost-effective alternatives to ablation, promoting health equity. This study investigates how ibutilide-mediated action potential prolongation enhances use-dependent effects of flecainide by shortening the diastolic interval, reducing drug unbinding, and suppressing atrial excitability to terminate re-entrant arrhythmia. Using computational modeling, we predict optimal sodium- and potassium-channel blocker combinations for chemical atrial defibrillation. The results suggest that acute flecainide-ibutilide application is a viable drug-repurposing strategy. Additionally, we assess the safety pharmacology of this combination on ventricular electrophysiology.
Clancy to present at the Division of Cardiovascular Sciences (DCVS) Strategic Vision Webinar Series

November 2024 — Colleen Clancy, Ph.D., professor and director of Precision Medicine and Data Sciences, has been invited to present in a virtual seminar series, “Strategic Vision Webinar Series”, hosted by the Division of Cardiovascular Sciences (DCVS). Clancy will present at the session dedicated to the Enhancing resilience. The topic of the presentation is "Revolutionizing Precision Medicine with Digital Twins: Prediction Through Computational Data Integration".
Clancy Presented at the Sixth Annual Cedar Sinai Precision Health Research Symposium

November 2024 — On November 6, 2024, Director Clancy joined other esteemed scientists to present at the sixth annual Cedar Sinai Precision Health Research Symposium. This year’s topic was focused on “Integrative Multi Mix and Translational Research for Precision Health”. It was a lively session with thrilling presentations about the future of precision medicine from Professors Steve Finkbeiner from The Gladstone and UCSF and Megan Hitchins from Cedars-Sinai. Spirited discussion during the moderated session.
2024 Nobel Prize Week Celebration


October 2024 — Professor Vladimir Yarov-Yarovoy (Associate Director of the Center for Precision Medicine and Data Sciences) attending the 2024 Nobel Prize Week celebration in Stockholm, Sweden. Professor David Baker (Professor Vladimir Yarov-Yarovoy’s postdoc and NIH Career Development grant mentor) received the 2024 Nobel Prize in Chemistry “for computational protein design”.
See also:
- https://www.nobelprize.org/prizes/chemistry/2024/press-release/
- https://www.nobelprize.org/prizes/chemistry/2024/baker/lecture/
- https://www.nobelprize.org/prizes/chemistry/2024/baker/facts/
Gonzalo Hernandez-Hernandez Recognized at UC Davis Chancellor’s Postdoctoral Reception

October 2024 — Gonzalo Hernandez-Hernandez has officially begun his UC Davis Chancellor’s Postdoctoral Fellowship through the University of California President’s Postdoctoral Fellowship Program. The fellowship provides scholars with exceptional professional development and faculty mentorship opportunities. Aimed at individuals with unique perspectives shaped by non-traditional educational backgrounds or a deep understanding of the experiences of historically underrepresented groups in higher education, the program works to enhance diversity within the academic community at the University of California.
Hernandez-Hernandez was recognized for his contributions to promoting diversity, including research focused on increasing equitable access in fields where women and minorities remain underrepresented. His project, titled “Predicting the Impact of Sex-Specific Differences in Vascular Smooth Muscle Cells in Mechanisms of Hypertension,” explores the development of tailored antihypertensive treatments based on sex. Guided by mentors Professor Fernando Santana and Professor Colleen Clancy, his work aims to advance precision medicine in hypertension treatment.
Gonzalo was honored during the Chancellor’s Postdoctoral Fellow reception, which kicked off Postdoctoral Appreciation Week, alongside his mentor, Professor Santana, and Provost Mary Croughan.
An artificial intelligence accelerated virtual screening platform for drug discovery
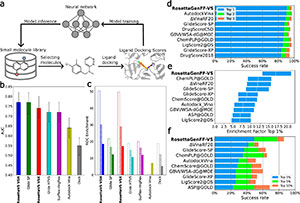
September 2024
Abstract
Structure-based virtual screening is a key tool in early drug discovery, with growing interest in the screening of multi-billion chemical compound libraries. However, the success of virtual screening crucially depends on the accuracy of the binding pose and binding affinity predicted by computational docking. Here we develop a highly accurate structure-based virtual screen method, RosettaVS, for predicting docking poses and binding affinities. Our approach outperforms other state-of-the-art methods on a wide range of benchmarks, partially due to our ability to model receptor flexibility. We incorporate this into a new open-source artificial intelligence accelerated virtual screening platform for drug discovery. Using this platform, we screen multi-billion compound libraries against two unrelated targets, a ubiquitin ligase target KLHDC2 and the human voltage-gated sodium channel NaV1.7. For both targets, we discover hit compounds, including seven hits (14% hit rate) to KLHDC2 and four hits (44% hit rate) to NaV1.7, all with single digit micromolar binding affinities. Screening in both cases is completed in less than seven days. Finally, a high resolution X-ray crystallographic structure validates the predicted docking pose for the KLHDC2 ligand complex, demonstrating the effectiveness of our method in lead discovery.
Colleen Clancy Presented the State of the Art Lecture at the ISHR-NAS Meeting

August 2024 — Colleen Clancy, Ph.D., professor and director of Precision Medicine and Data Sciences, presented the state of the art lecture about her recent work on computational digital twins at the International Society for Heart Research (ISHR) - NAS meeting.
Fred Meyers Begins Term as Chair of City Year Sacramento Board

August 2024 — Fred Meyers, M.D., Distinguished Professor Emeritus of Internal Medicine/Hematology-Oncology at UC Davis School of Medicine, has begun his term as Board Chair for City Year Sacramento (CYSac). CYSac, an AmeriCorps education non-profit, recruits young adults for a year of service as Student Success Coaches (SSCs) in under-resourced schools within the Oak Park and South Sacramento areas of the Sacramento City Unified School District.
"My passion for City Year Sacramento stems from its mission to embrace education as the surest path to good health for individuals and our communities," said Fred Meyers.
Advances in Induced Pluripotent Stem Cell-derived Cardiac Myocytes: Technological Breakthroughs, Key Discoveries and New Applications
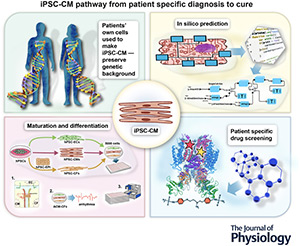
July 2024
Abstract
A transformation is underway in precision and patient-specific medicine. Rapid progress has been enabled by multiple new technologies including induced pluripotent stem cell-derived cardiac myocytes (iPSC-CMs). Here, we delve into these advancements and their future promise, focusing on the efficiency of reprogramming techniques, the fidelity of differentiation into the cardiac lineage, the functional characterization of the resulting cardiac myocytes, and the many applications of in silico models to understand general and patient-specific mechanisms controlling excitation–contraction coupling in health and disease. Furthermore, we explore the current and potential applications of iPSC-CMs in both research and clinical settings, underscoring the far-reaching implications of this rapidly evolving field.
Structure-Activity Relationship Study Identifies a Novel Lipophilic Amiloride Derivative that Efficiently Kills Chemoresistant Breast Cancer Cells
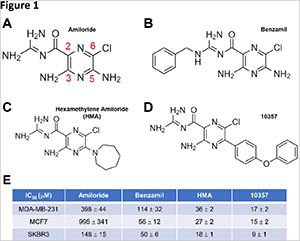
July 2024
Abstract
Derivatives of the potassium-sparing diuretic amiloride are preferentially cytotoxic toward tumor cells relative to normal cells, and have the capacity to target tumor cell populations resistant to currently employed therapeutic agents. However, a major barrier to clinical translation of the amilorides is their modest cytotoxic potency, with estimated IC50 values in the high micromolar range. Here we report the synthesis of ten novel amiloride derivatives and the characterization of their cytotoxic potency toward MCF7 (ER/PR-positive), SKBR3 (HER2-positive) and MDA-MB-231 (triple negative) cell line models of breast cancer. Comparisons of derivative structure with cytotoxic potency toward these cell lines underscore the importance of an intact guanidine group, and uncover a strong link between drug-induced cytotoxicity and drug lipophilicity. We demonstrate that our most potent derivative called LLC1 is preferentially cytotoxic toward mouse mammary tumor over normal epithelial organoids, acts in the single digit micromolar range on breast cancer cell line models representing all major subtypes, acts on cell lines that exhibit both transient and sustained resistance to chemotherapeutic agents, but exhibits limited anti-tumor effects in a mouse model of metastatic breast cancer. Nonetheless, our observations offer a roadmap for the future optimization of amiloride-based compounds with preferential cytotoxicity toward breast tumor cells.
Association of Neighborhood and Environmental Factors With Clinical Phenotypes and Outcomes in Heart Failure With Preserved Ejection Fraction
July 2024 — Heart failure with preserved ejection fraction (HFpEF) is heterogeneous with multiple comorbidities and limited therapeutic options.1 Multiple pathologies contribute to the development of distinct clinical HFpEF phenogroups. Evidence suggests that social determinants of health (SDoH) are pivotal in the pathogenesis of cardiovascular disease. Defined by the Centers for Disease Control and Prevention and the World Health Organization, SDoH refers to the conditions in the environments where people are born, live, learn, work, play, worship, and age, influencing health outcomes and quality-of-life risks. SDoH encompasses 5 domains: (1) Education Access and Quality, (2) Economic Stability, (3) Social and Community Context, (4) Health Care Access and Quality, (5) Neighborhood and Built Environment.
New Extramural Funding
July 2024 — New extramural funding including NIH grant R01HL128537, “Digital Twins from the Atom to the Rhythm” (R01), and R01HL152681 “Metabolic Control of Cardiac Pacemaking” (R01) from the National Institutes of Health. Heart, Blood and Lung Institute, have been awarded.
Toward High-resolution Modeling of Small Molecule–ion Channel Interactions
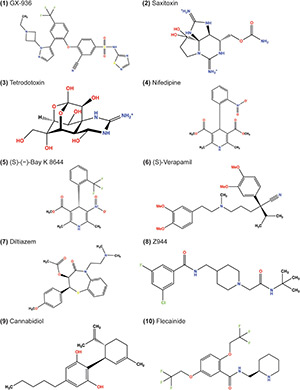
June 2024
Abstract
Ion channels are critical drug targets for a range of pathologies, such as epilepsy, pain, itch, autoimmunity, and cardiac arrhythmias. To develop effective and safe therapeutics, it is necessary to design small molecules with high potency and selectivity for specific ion channel subtypes. There has been increasing implementation of structure-guided drug design for the development of small molecules targeting ion channels. We evaluated the performance of two RosettaLigand docking methods, RosettaLigand and GALigandDock, on the structures of known ligand–cation channel complexes. Ligands were docked to voltage-gated sodium (NaV), voltage-gated calcium (CaV), and transient receptor potential vanilloid (TRPV) channel families. For each test case, RosettaLigand and GALigandDock methods frequently sampled a ligand-binding pose within a root mean square deviation (RMSD) of 1–2 Å relative to the experimental ligand coordinates. However, RosettaLigand and GALigandDock scoring functions cannot consistently identify experimental ligand coordinates as top-scoring models. Our study reveals that the proper scoring criteria for RosettaLigand and GALigandDock modeling of ligand–ion channel complexes should be assessed on a case-by-case basis using sufficient ligand and receptor interface sampling, knowledge about state-specific interactions of the ion channel, and inherent receptor site flexibility that could influence ligand binding.
Reaching Across the Causeway Award 2024
Co-PIs: Kermit L. Carraway, James M. Angelastro
2024
Abstract
The non-canonical (β-catenin-independent) Wnt/planar cell polarity (Wnt/PCP) pathway plays central roles in embryonic development by mediating cellular motility events required for proper tissue structuring. Accumulating evidence suggests that the Wnt/PCP pathway is exploited by some solid tumors to promote their growth and invasiveness. Glioblastoma (GBM) is the most common and lethal form of brain cancer in humans. Efforts to thwart GBM malignancy with therapeutic agents targeting presumed oncogenic drivers have not been successful, suggesting that unknown or unexplored pathways might additionally contribute to malignancy. We have found that key components of the Wnt/PCP pathway are overexpressed in GBM relative to normal brain tissue independent of GBM subtype, and overexpression correlates with poorer patient outcomes. Moreover, we have observed that suppression of Fzd7 and Vangl1, core components of the Wnt/PCP signaling complex, reduce GBM cell growth and invasiveness in vitro and in vivo. Together these observations suggest that aberrant Wnt/PCP engagement may contribute to the malignant properties of a wide range of GBM patients’ tumors. The driving hypothesis for the project is that Wnt/PCP signaling contributes to the malignant properties of GBM largely independent of specific subtypes and suspected molecular drivers, and that mechanistic vulnerabilities may be identified that could ultimately aid in the development of novel therapeutic strategies and agents. Our overarching aims for the project are to validate Wnt/PCP as a viable target for multiple GBM subtypes using a series of patient-derived xenograft (PDX) models, and to develop a more thorough mechanistic understanding of the interactions among pathway components. In Aim 1 we will assess the Wnt/PCP dependence of five patient-derived xenograft models of primary GBM from the Mayo Brain Tumor PDX National Resource that represent an array of oncogenic drivers using cellular proliferation and invasiveness assays. In addition, we will determine the impact of Wnt/PCP component ablation on the growth of orthotopically xenografted tumors derived from the five genetically diverse PDX lines in NOD SCID mice, as well as in tumors from a retrovirus-induced model of GBM in immune-intact mice. We anticipate that these studies will begin to highlight the GBM subtype-agnostic nature of Wnt/PCP dependence. In Aim 2, we will unravel the molecular mechanisms underlying Wnt/PCP-induced motility/invasiveness and proliferation. Specifically, we will assess the role of a newly identified Vangl1/Fzd7 complex in signaling to the actin cytoskeleton, and examine the mechanism of Wnt/PCP-induced cross-regulation of the Akt kinase in mediating GBM cell proliferation. The successful completion of the proposed studies will validate Wnt/PCP as target for multiple GBM subtypes, and will develop a mechanistic platform upon which Wnt-PCP targeting therapeutics may ultimately be developed.
A Computational Model Predicts Sex-specific Responses to Calcium Channel Blockers in Mammalian Mesenteric Vascular Smooth Muscle
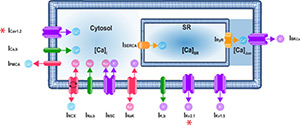
February 2024
Abstract
The function of the smooth muscle cells lining the walls of mammalian systemic arteries and arterioles is to regulate the diameter of the vessels to control blood flow and blood pressure. Here, we describe an in silico model, which we call the 'Hernandez-Hernandez model', of electrical and Ca2+ signaling in arterial myocytes based on new experimental data indicating sex-specific differences in male and female arterial myocytes from murine resistance arteries. The model suggests the fundamental ionic mechanisms underlying membrane potential and intracellular Ca2+ signaling during the development of myogenic tone in arterial blood vessels.
Elucidating Molecular Mechanisms of Protoxin-II State-specific Binding to the Human NaV1.7 Channel
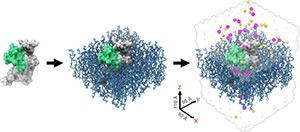
February 2024
Abstract
Human voltage-gated sodium (hNaV) channels are responsible for initiating and propagating action potentials in excitable cells, and mutations have been associated with numerous cardiac and neurological disorders. hNaV1.7 channels are expressed in peripheral neurons and are promising targets for pain therapy. The tarantula venom peptide protoxin-II (PTx2) has high selectivity for hNaV1.7 and is a valuable scaffold for designing novel therapeutics to treat pain. Here, we used computational modeling to study the molecular mechanisms of the state-dependent binding of PTx2 to hNaV1.7 voltage-sensing domains (VSDs).
UC Davis Health Team Uses AI to Predict Risk of Liver Cancer
New algorithms developed by clinicians and data scientists could help personalize treatment
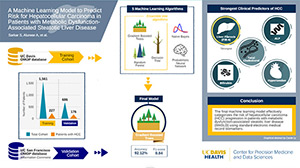
February 2024 — A team of UC Davis Health clinicians and data scientists have developed a machine-learning model to better predict which patients are at greater risk of developing a common type of liver cancer, hepatocellular carcinoma (HCC).
The findings of their research — published in the journal Gastro Hep Advances — describe how predictive-learning can aid physicians in providing early HCC risk assessments for patients diagnosed with metabolic dysfunction-associated steatotic liver disease, or MASLD. The pilot technology may be able to give physicians critical information to screen patients more closely and thus offer more personalized care.
Toward Digital Twin Technology for Precision Pharmacology
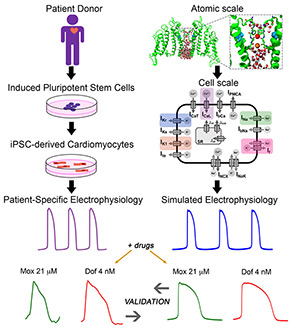
February 2024
Abstract
The authors demonstrate the feasibility of technological innovation for personalized medicine in the context of drug-induced arrhythmia. The authors use atomistic-scale structural models to predict rates of drug interaction with ion channels and make predictions of their effects in digital twins of induced pluripotent stem cell-derived cardiac myocytes. The authors construct a simplified multilayer, 1-dimensional ring model with sufficient path length to enable the prediction of arrhythmogenic dispersion of repolarization. Finally, the authors validate the computational pipeline prediction of drug effects with data and quantify drug-induced propensity to repolarization abnormalities in cardiac tissue. The technology is high throughput, computationally efficient, and low cost toward personalized pharmacologic prediction.
2023
Structural Modeling of Ion Channels Using AlphaFold2, RoseTTAFold2, and ESMFold
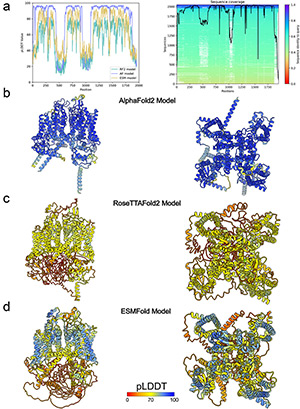
September 2023
Abstract
Ion channels play key roles in human physiology and are important targets in drug discovery. The atomic-scale structures of ion channels provide invaluable insights into a fundamental understanding of the molecular mechanisms of channel gating and modulation. Recent breakthroughs in deep learning-based computational methods, such as AlphaFold, RoseTTAFold, and ESMFold have transformed research in protein structure prediction and design. We review the application of AlphaFold, RoseTTAFold, and ESMFold to structural modeling of ion channels using representative voltage-gated ion channels, including human voltage-gated sodium (NaV) channel - NaV1.8, human voltage-gated calcium (CaV) channel – CaV1.1, and human voltage-gated potassium (KV) channel – KV1.3. We compared AlphaFold, RoseTTAFold, and ESMFold structural models of NaV1.8, CaV1.1, and KV1.3 with corresponding cryo-EM structures to assess details of their similarities and differences. Our findings shed light on the strengths and limitations of the current state-of-the-art deep learning-based computational methods for modeling ion channel structures, offering valuable insights to guide their future applications for ion channel research.
A Multiscale Predictive Digital Twin for Neurocardiac Modulation
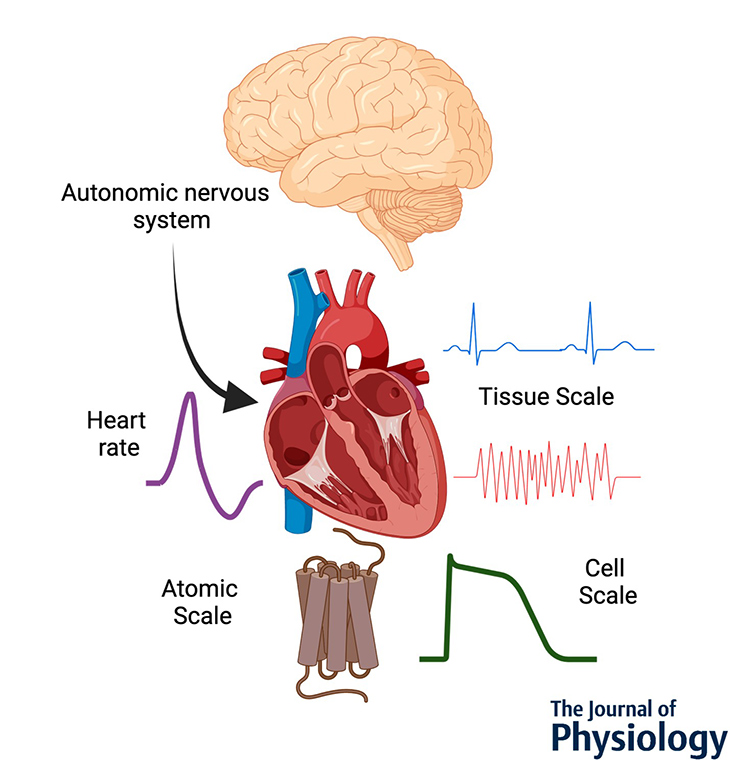
August 2023
Abstract
Cardiac function is tightly regulated by the autonomic nervous system (ANS). Activation of the sympathetic nervous system increases cardiac output by increasing heart rate and stroke volume, while parasympathetic nerve stimulation instantly slows heart rate. Importantly, imbalance in autonomic control of the heart has been implicated in the development of arrhythmias and heart failure. Understanding of the mechanisms and effects of autonomic stimulation is a major challenge because synapses in different regions of the heart result in multiple changes to heart function. For example, nerve synapses on the sinoatrial node (SAN) impact pacemaking, while synapses on contractile cells alter contraction and arrhythmia vulnerability. Here, we present a multiscale neurocardiac modelling and simulator tool that predicts the effect of efferent stimulation of the sympathetic and parasympathetic branches of the ANS on the cardiac SAN and ventricular myocardium. The model includes a layered representation of the ANS and reproduces firing properties measured experimentally. Model parameters are derived from experiments and atomistic simulations. The model is a first prototype of a digital twin that is applied to make predictions across all system scales, from subcellular signalling to pacemaker frequency to tissue level responses. We predict conditions under which autonomic imbalance induces proarrhythmia and can be modified to prevent or inhibit arrhythmia. In summary, the multiscale model constitutes a predictive digital twin framework to test and guide high-throughput prediction of novel neuromodulatory therapy.


