Q&A with Director of Aortic Surgery Steven Maximus on newly approved thoracic branch endoprosthesis
The new medical prosthesis is making repairs to the thoracic aorta easier and safer for patients
For many years, the most common surgical method for repairing a damaged thoracic aorta involving the left subclavian artery was either open repair or a hybrid procedure with left carotid to subclavian bypass and TEVAR. However, a new technological breakthrough is making repairs to the aorta easier, with faster patient recoveries for some.
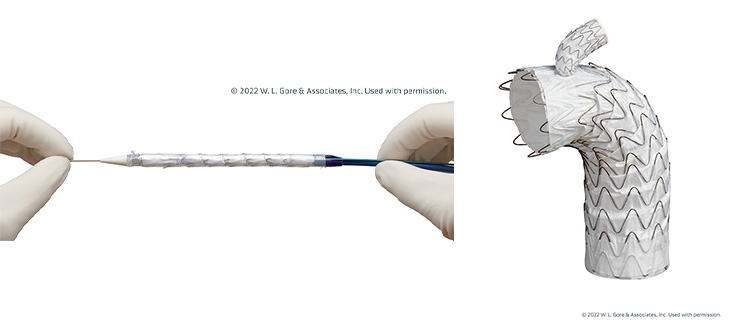
The GORE® TAG® Thoracic Branch Endoprosthesis is a stent graft approved by the FDA in 2022 to support damaged aorta tissue. UC Davis Health was the first University of California health system to implant the device since it was approved and is now one of the top three medical centers in the country placing it.
UC Davis Health Director of Aortic Surgery Steven Maximus is one of the leading surgeons placing the device. He’s also an assistant professor and GORE® consultant. “It’s exciting,” he said. “We’ve had a number of aortic dissections where the patient probably would have had poor outcomes or prolonged procedures if we hadn’t used it.”
Maximus has performed 50 surgeries with the endoprosthesis since September 2022.
In this Q&A, he’s sharing his extensive knowledge and best practices for using the device.
What is the GORE® TAG® Thoracic Branch Endoprosthesis and what does it do?
It's a branched stent graft and the only FDA-approved branched TEVAR device indicated for zone two in the thoracic aorta. It's indicated for usage in Zone 2 thoracic aortic aneurysms, dissections, and ruptures.
In the case of aneurysms, instead of undergoing hybrid repair, you can place the stent to reroute the blood flow and exclude the aneurysm. During aortic dissections, we’re able to restore blood flow to both the left subclavian and the thoracic aorta using the system. We're basically realigning a patient’s pipes from the inside instead of making a large incision in the chest or the neck.
How do you place the stent?
You obtain percutaneous access sites in the left radial artery and either groin and deliver the device through the common femoral artery and the iliac arteries. We recommend two surgeons for the procedure because of the two access sites.
What kinds of patients are best suited for these devices?
The on-label use is injury that starts distal to zone two of the aorta. Most of our patients that we treat have issues from smoking or uncontrolled hypertension. It’s probably not good for patients who have aneurysms or dissections due to connective tissue disorders.
There are also anatomic restrictions. We measure based on anatomy, and not everyone is a candidate for this device. People who have allergies to the materials or active infections causing aortic damage would also be excluded from using the stent.
What are the guidelines for placing them?
You need adequate arterial access to accommodate a large sheath, which is how you deliver the device through the arteries. Usually the smallest size is a 22 French sheath, and up to 26 for the larger sheath sizes. You must have an adequate seal zone (usually 2cm) meaning an area of normal proximal and distal aorta for the device to seal. The largest graft comes in 45 mm diameter, which treats a 34-42 diameter aorta.
Are there any drawbacks?
There is the risk of stroke. There's always a risk that we can cause further injury to the artery with a retrograde dissection. We can crack the artery into the aortic arch more proximally and cause problems in even the heart itself. That hasn't happened to us yet, but you have to make sure you're using the right size device.
What’s the average recovery time for the procedure?
For the patients who get this done electively, most go home the next day.
Is there anything else you’d like physicians to know about using the stent?
We have expanded the use of this device over time. We’ve performed a few endovascular zone 0 aortic repairs and have had good success with this. In this case, the branch is to the innominate artery, and the patient undergoes cervical debranching with a carotid-carotid bypass and left subclavian bypass. This is off-label use, but it is a possible option for patients who would not tolerate surgical repair that would otherwise require open heart surgery with cardiopulmonary bypass.
The UC Davis Vascular Center is a national leader in addressing vascular disease, including peripheral artery disease, cerebrovascular disease and diabetes-associated vascular issues. The center's board-certified surgeons, clinicians and researchers offer comprehensive care using a team approach that emphasizes prevention, early diagnosis and minimally invasive treatments. Through state-of-the-art technology and a strong focus on continuity of care, patients receive the best in both technical support and follow-up treatment. Collaborations between the center's experts and UC Davis stem cell investigators are leading to new cellular therapies that generate new arteries and restore blood flow in diseased legs and arms, reducing the need for amputations. For more information, visit health.ucdavis.edu/vascular.