Examples of Allen Gao's Research
Current research programs
- Signaling transduction pathways mediated by cytokines and androgen receptor in androgen-recurrent prostate cancer
- Mechanisms of selenium chemoprevention and therapy
- Identify factors associated with prostate bone metastasis
- Targeting cell signaling pathways in prostate cancer
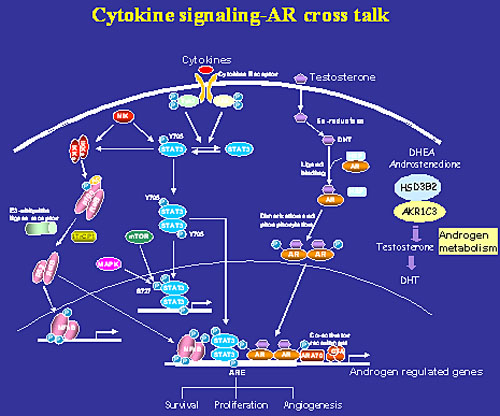
Molecular mechanisms of prostate cancer transition from androgen-dependence to androgen-independence.
Most prostate cancer patients respond initially to androgen ablation and antiandrogen therapy. However, virtually all patients will relapse due to acquisition of the growth of androgen-independent tumor cells. Unfortunately, there is currently no effective treatment for men with androgen-independent prostate cancer. The molecular changes in prostate cancer cells lead to the androgen-independent growth are incompletely understood. One of my research focuses is to understand intracellular signaling regulations in prostate cancer cells leading to androgen-independence. We have identified cytokines such as IL-6 and IL-4 as inducers of androgen-independent progression of prostate cancer cells. The roles of signaling pathways such as Stat3, Akt and NF-κB induced by IL-6 and IL-4 have been subsequently demonstrated in androgen receptor activation and progression of androgen- independent prostate cancer. Currently, the role of these signal pathways (Stat3, NF-κB, Akt) and coregulators (CBP/p300 and TIF) in androgen receptor activation and androgen recurrent prostate cancer are under intensive investigation.
We have established several novel prostate cancer cell lines representing androgen recurrent prostate cancer progression including LNCaP-IL-6+ and LNCS cell lines. LNCS is an androgen-deprivation induced and androgen suppressed human prostate cancer cell line, LNCS. This cell line will be used as a model system to dissect molecular mechanisms of androgen recurrent progression since it mimics clinical progression of androgen recurrent prostate cancer. In addition, it will be a model cell line to understand the mechanism of intermittent androgen suppression widely used for treatment of advanced prostate cancer. We have analyzed the global gene expression profile by microarray analysis and protein expression profiles by 2D-gel and proteomic analysis and identified several genes involved in recurrent prostate cancer. More detailed analysis of these genes involved in recurrent prostate cancer progression is currently under investigation.
In addition, we have focused our research on understanding interactions among individual signaling transduction pathways (cross-talk). We have made a novel finding that Stat3 activates NF-kB p100 processing involves CBP/p300-mediated acetylation. We found that both Stat3 activation and NF-kB p52 overexpression play critical roles in androgen independent progression.
Mechanisms of selenium chemoprevention and therapy
Prevention trials demonstrated that selenium reduced prostate cancer incidence by 50%, establishing selenium as a promising chemopreventive agent for prostate cancer. We made several novel discoveries on molecular basis of selenium anticancer activity in prostate and breast cancer. We found that selenium down regulates androgen receptor activation in prostate cancer cells, and differentially regulates the expression of estrogen receptors in breast cancer cells. These novel findings provide molecular basis and rationale for selenium chemoprevention and therapy for prostate cancer and breast cancer. Our goals are to elucidate the importance of alteration of androgen and estrogen signaling pathways by selenium in prostate and breast cancer, and explore the potential applications of selenium either alone or combinations with chemotherapeutic agents in prostate cancer chemoprevention and therapy.
In addition to disrupt androgen receptor signaling, we found that selenium also inhibits survivin expression by preventing Sp1 binding to its promoter. We identified that selenium prevents the binding of Sp1 or Sp1-like proteins to the 37-bp cis-acting DNA element in the survivin promoter. Furthermore, inhibition of survivin expression by siRNA enhanced selenium’s inhibitory effects on cell growth. Whereas, overexpression of survivin in LNCaP human prostate cancer cells desensitized cancer cells to selenium effect, suggesting that the expression of survivin plays an important role in determining the response of cancer cells to selenium. Our findings suggest that selenium down-regulated survivin expression by preventing the binding of Sp1 or Sp1-like proteins to the promoter of survivin, which contributes at least in part to the inhibitory effect of selenium on survivin gene transcription. In addition, down-regulation of survivin expression may account for one of the molecular mechanisms of the anti-cancer effects of selenium.
Identify factors associated with prostate bone metastasis
Bone is the frequent site of many types of cancer metastasis including prostate cancer. Advanced prostate cancer is frequently accompanied by the development of unique bone metastases characterized as to be osteoblastic (bone forming), which results in significant complications including bone pain, fractures and spinal-cord compression even hemiparesis, leading to morbidity with no curable treatment. The high prevalence of osteoblastic bone metastases in prostate cancer involves the production of osteoblast-stimulating factors by prostate cancer cells. One of our research focuses is to identify factors involved in prostate induced osteoblasts. Several factors have been identified including prostate specific antigen (PSA). PSA is a serine protease uniquely produced by prostate cancer cells and is an important serological marker for prostate cancer. Our findings by examining global gene expression changes using cDNA arrays demonstrated that PSA in an inducer of osteoblast differentiation. Modulation of the expression of osteogenic genes and alteration of the balance between OPG-RANKL by PSA suggests that PSA produced by metastatic prostate cancer cells may participate in bone remodeling in favor of development of osteoblastic metastases in the heterogeneous mixture of osteolytic and osteoblastic lesions. These findings provide a molecular basis for understanding the high prevalence of osteoblastic bone metastases in prostate cancer. We are now focusing on targeting osteogenic factors including PSA for bone metastatic prostate cancer.
We have cloned RANKL promoter and identified critical regions and a key transcription factor that may be responsible for RANKL transcriptional regulation. The roles of RANKL and the identified transcription factor that regulates RANKL expression in bone remodeling and prostate cancer bone metastasis are the focus of our current studies.
Targeting cell signaling pathways in prostate cancer
Our objective is to evaluate the anti-tumor efficacy of targeting signaling transduction pathways identified critically involved in the development and progression of prostate cancer in preclinical models with the ultimate aim of designing novel therapeutic strategies for prostate cancer. We are currently using high through-put screening to identify small molecule inhibitors and using lentiviral/adenoviral delivering siRNAs to target IL-6/Stat3, androgen receptor, NF-kB and Akt signaling pathways, surviving and RANKL signaling pathways. We have identified several potential inhibitors for Stat3, NF-kB and survivin, and their therapeutic potencies are currently under investigation in both cell culture and animal models.

