UC Davis launches stem cell study to reduce amputations from vascular disease and diabetes
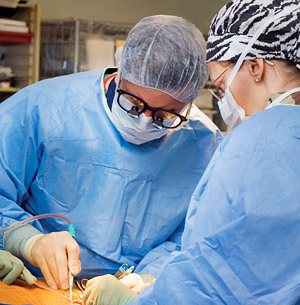
The UC Davis Vascular Center is currently the only West Coast site for a study to determine if a patient's own stem cells can be used to help generate new blood vessels and reduce the need for amputations due to advanced vascular disease.
Posted March 30, 2011
UC Davis Vascular Center researchers have embarked on a highly anticipated study that involves using a patient’s own stem cells to increase blood circulation to the lower leg with the hope of preventing amputation due to severe arterial disease or diabetes.
“Losing a limb is a devastating complication of advanced vascular disease,” said John Laird, medical director of the vascular center and a principal investigator of the study. “We are deeply committed to finding alternatives to amputations that save lives and improve quality of life for vascular patients.”
An estimated 85,000 leg amputations are performed each year in the U.S. due to advanced atherosclerosis — also known as critical limb ischemia — which occurs when the buildup of fatty deposits, calcium and plaque in arteries greatly reduces blood flow to lower extremities. Current treatments for the condition include opening blockages with balloon angioplasty, bolstering weakened arteries with metal stents or bypassing damaged arteries with vein grafts. When the disease progresses to the point of limb-threatening ischemia and when angioplasty, stents or surgery are not viable, amputation becomes the only option.
 “We are deeply committed to finding alternatives to amputations that save lives and improve quality of life for vascular patients.”
“We are deeply committed to finding alternatives to amputations that save lives and improve quality of life for vascular patients.”
— John Laird
“Many patients require limb amputation because angioplasty or surgery is either not successful or not possible,” said Laird. “Stem cell therapies could provide less-invasive options for these desperate patients and may offer a more permanent way to restore circulation.”
The study involves a one- to two-hour surgical procedure during which bone marrow is harvested from the pelvis and spun in a centrifuge to separate mononuclear cells. These cells consist of white blood cells and mononuclear stem cells containing concentrations of endothelial progenitor cells — the stem cells responsible for initially forming blood vessels in utero. The separated stem cells are injected at multiple points into the muscle of the leg at risk for amputation. The cells are also tested for sterility and quality in the new UC Davis Good Manufacturing Practice Facility that was funded by the California Institute for Regenerative Medicine (CIRM).
“The hope is that what these cells do in the very early stages of life can be repeated much later in life by producing new blood vessels that circumvent damaged ones altogether,” said Laird.
About the UC Davis Vascular Center:
 The UC Davis Vascular Center is a national leader in addressing vascular disease, including peripheral artery disease, cerebrovascular disease and diabetes-associated vascular issues. The center’s board-certified surgeons, clinicians and researchers utilize a comprehensive care and team-treatment approach that emphasizes prevention, early diagnosis and minimally invasive treatments. Through state-of-the-art technology and a strong continuity-of-care philosophy, patients are assured the best in both technical support and follow-up treatment. Additional information is available on the center's website.
The UC Davis Vascular Center is a national leader in addressing vascular disease, including peripheral artery disease, cerebrovascular disease and diabetes-associated vascular issues. The center’s board-certified surgeons, clinicians and researchers utilize a comprehensive care and team-treatment approach that emphasizes prevention, early diagnosis and minimally invasive treatments. Through state-of-the-art technology and a strong continuity-of-care philosophy, patients are assured the best in both technical support and follow-up treatment. Additional information is available on the center's website.
About the UC Davis Stem Cell Program:
 UC Davis is playing a leading role in regenerative medicine, with nearly 150 scientists working on research projects focused on turning stem cells into cures. The 91,000 square-foot facility that houses the UC Davis Institute for Regenerative Cures — supported by the California Institute for Regenerative Medicine — is the university's hub for stem cell science. It includes Northern California's largest academic Good Manufacturing Practice facility, with state-of-the-art equipment and manufacturing rooms for cellular and gene therapies. For more information, visit the program website.
UC Davis is playing a leading role in regenerative medicine, with nearly 150 scientists working on research projects focused on turning stem cells into cures. The 91,000 square-foot facility that houses the UC Davis Institute for Regenerative Cures — supported by the California Institute for Regenerative Medicine — is the university's hub for stem cell science. It includes Northern California's largest academic Good Manufacturing Practice facility, with state-of-the-art equipment and manufacturing rooms for cellular and gene therapies. For more information, visit the program website.
The entire process requires one operating room procedure and involves minimal discomfort. Patients remain in the hospital overnight and are expected to return to the UC Davis Vascular Center in Sacramento, Calif., for a series of five follow-up visits over the course of a year.
The trial sponsor, Biomet Biologics of Warsaw, Ind., manufactures the specialized equipment — called MarrowStim™ — used to extract blood cells from bone marrow along with the high-speed, table-top centrifuge that separates and concentrates the mononuclear cells, making it easy to deliver them to treatment targets. The company recently completed a Phase I safety study of the technology, and the results were used to refine the technology and launch the new national FDA‐approved trial.
 “This next stage of our research will determine if the treatment truly offers hope for people without other options and who are at risk of losing a limb.”
“This next stage of our research will determine if the treatment truly offers hope for people without other options and who are at risk of losing a limb.”
— Jan Nolta
The study initiates the stem cell research program of the vascular center, which is currently the only West Coast site for this investigation. UC Davis was selected to participate because of its vascular and bone-marrow harvest expertise in addition to the resources of its Institute for Regenerative Cures, where team-oriented science is advancing breakthrough discoveries in stem cell therapies.
“Our own research in mice has shown that adult human stem cells are very efficient at targeting areas of low oxygen and promoting the formation of new blood vessels,” said Jan Nolta, director of the UC Davis Stem Cell Program and Institute for Regenerative Cures. “This next stage of our research will determine if the treatment truly offers hope for people without other options and who are at risk of losing a limb.”
Laird and Nolta carefully planned the study with a team of surgeons, researchers, nurses and lab specialists from the Institute for Regenerative Cures and UC Davis Medical Center.
For information on study enrollment criteria, contact Christy Pifer, manager of UC Davis Vascular Center clinical research, at 916-734-4156.