Alae Yaseen, M.D., M.S., Surgical Pathology Fellow
John Bishop, M.D., Health Sciences Clinical Professor, Vice Chair for Clinical Services, Department of Pathology and Laboratory Medicine, UC Davis
Background
Human epidermal growth factor receptor-2 (HER2) is a proto-oncogene that encodes a tyrosine kinase receptor belonging to the epidermal growth factor receptor (EGFR) family. The HER2 signaling pathway is involved in cell division, proliferation, differentiation, and apoptosis(1).
A subset of Gastroesophageal Adenocarcinomas (7% to 38%) overexpress HER2. The overexpression varies with location (gastroesophageal junction greater than gastric type), differentiation (well-moderately differentiated greater than poorly differentiated) and histologic type (Gastric intestinal-type greater than diffuse-type) (2).
HER2 overexpression is usually caused by gene amplification, and like breast carcinomas, HER2 overexpression in GEA has a therapeutic utility. Treatment with anti-HER2 monoclonal antibody trastuzumab (Herceptin®️, Genentech) is recommended for patients with inoperable, locally advanced, recurrent, or metastatic gastric or gastroesophageal junction adenocarcinoma. This treatment significantly increases survival when compared to patient treated with chemotherapy alone (3).
Discussion
Assessment for tumor HER2 overexpression using immunohistochemistry (IHC) or in situ hybridization (ISH) is recommended by the National Comprehensive Cancer Network (NCCN) (3). HER2 status can be assessed using either biopsy or surgical resection materials. However, HER2 immunostaining in GEA is more variable and doesn’t demonstrate the complete membranous staining which is expected in HER2-positive breast cancers. Instead, GEA often exhibit a basolateral staining pattern. On the other hand, detection of HER2 gene amplification by FISH is similar to that in breast cancer and is assessed according to the (ASCO/CAP) 2013 guidelines (4).
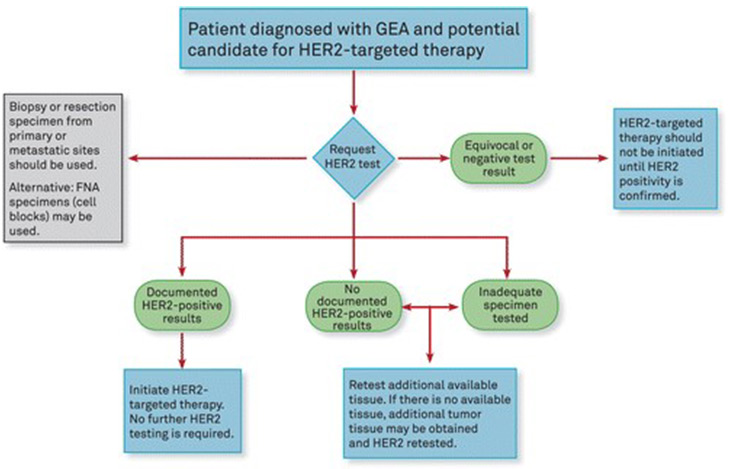
Algorithm for clinicians. Abbreviations: FNA, fine-needle aspiration; GEA, gastroesophageal adenocarcinoma; HER2, human epidermal growth factor receptor 2. Guideline From the CAP College of American Pathologists, ASCP American Society for Clinical Pathology, and ASCO American Society of Clinical Oncology (1).
The NCCN guideline for gastric HER2 biomarkers recommends that IHC for HER2 should be performed first and reported using the modified scoring system proposed by Hofmann et al. and used in the ToGA trial. See table below (5, 6). It’s also recommended that the pathologists should select tissue areas of lowest grade tumor, or those with intestinal morphology as they are more likely to demonstrate the HER2-positive results.
If the immunohistochemical results are equivocal (2+), in-situ hybridization studies (ISH) should be performed. This is only applicable for HER2 IHC with (2+ score). Negative and positive IHC (scores of 0, 1+, or 3+) do not require further ISH testing.
HER2 IHCScoreHER2 IHC Pattern inSurgical SpecimenHER2 IHC Pattern in BiopsySpecimenHER2 ExpressionAssessment0No reactivity or membranous reactivity in <10% of cancer cellsNo reactivity or no membranous reactivity in any cancer cellNegative by IHC1+Faint or barely perceptible membranous reactivity in ≥10% of cancer cells; cells are reactive only in part of their membraneCancer cell cluster (≥5 neoplastic cells) with a faint or barely perceptible membranous reactivity irrespective of percentage of cancer cells positiveNegative by IHC2+Weak to moderate complete, basolateral or lateral membranous reactivity in ≥10% of tumor cellsCancer cell cluster (≥5 neoplastic cells) with a weak to moderate complete, basolateral, or lateral membranous reactivity irrespective of percentage of cancer cells positiveEquivocal by IHC3+Strong complete, basolateral or lateral membranous reactivity in ≥10% of cancer cellsCancer cell cluster (≥5 neoplastic cells) with a strong complete basolateral, or lateral membranous reactivity irrespective of percentage of cancer cellsPositive by IHC
The guidelines from the CAP, ASCP and ASCO provide 11 recommendations for clinicians and pathologists to handle the testing of HER2 in GEA. Beyond those previously discussed above, the guidelines include recommendations for the clinician to request the HER2 testing on tumor tissue before starting treatment in patients with advanced GEA. this can be done on biopsy or resection material, and in primary or metastatic tumor. The laboratories/pathologists should specify and validate the appropriate assays for HER2 testing and reporting.
References
- Bartley, AN, Washington, MK, Ventura, CB et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Arch Pathol Lab Med. 2016 Dec; 140(12):1345-1363.
- Ajani JA, D’Amico TA, Almhanna K, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Gastric Cancer, version 3.2015. National Comprehensive Cancer Network, Inc.
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697.
- Wolff AC, Hammond MEH, Allision KH, et al. HER2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Oncol Pract. 2018 Jul;14(7):437-441.
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet. 2010;376(9742):687-697.
- Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11(5):531-546.



