Nam Tran, Ph.D.; Samer Albahra, M.D.
INTRODUCTION
The nasopharyngeal (NP) swab serves as the primary specimen type for respiratory molecular pathogen detection.1 During the novel coronavirus infectious disease (COVID) pandemic of 2019, the NP swab became the centerpiece for detecting the severe acute respiratory syndrome – coronavirus – 2 (SARS-CoV-2). Unfortunately, NP swab collection can be extremely uncomfortable for patients. Combined with swab shortages observed early in the pandemic, many facilities pursued alternate specimen types such as saliva.2 Now, over a year later, as supply chains improve, the use of oropharyngeal (OP), mid-turbinate (MT) and anterior nares (AN) swab are now commonly used for COVID-19 testing.
LABORATORY BEST PRACTICE
The Infectious Disease Society of America (IDSA) recommends the use of NP swab, MT swab, AN swab, saliva or a combined AN/OP swab rather than an OP swab alone for SARS-CoV-2 RNA testing in symptomatic individuals suspected of having COVID-19.1 This recommendation comes with several caveats. Studies show that NP, MT, and AN swabs are comparable. Other studies suggest MT and AN may lose some sensitivity compared to NP specimens, with AN having a relative sensitivity ranging from 82 to 88%.3 AN achieves the highest concordance with NP when viral load is >1,000 RNA copies/mL4. Saliva specimens also exhibits good performance; however, this complex specimen type may create more variability due to inconsistent production of saliva by patients, and the potential for variable viscosity due to hydration status or other factors.5 Oropharyngeal swab, despite being more tolerated by patients, are the least desirable since data suggests this specimen type exhibits a higher false negative rate.1 To this end, UC Davis Health has approved only NP and AN swabs for SARS-CoV-2 polymerase chain reaction (PCR) testing at this time. The AN swab option provides a less invasive alternative for collection, with NP swab still being recommended. Saliva and MT swab samples are not approved at our institution.
PRE-ANALYTIC FACTORS IMPACTING COVID-19 TEST PERFORMANCE
In addition to the specimen type, it is worth discussing pre-analytic factors influencing SARS-CoV-2 testing performance.6 Specifically, the majority of factors impacting SARS-CoV-2 detection occurs before the test itself. Factors such as:
- Specimen collection quality: How well a healthcare professional collects any swab sample influences the amount of SARS-CoV-2 for testing. Even with a perfectly sensitive and specific PCR test, the lack of SARS-CoV-2 RNA on a swab results in a negative result regardless of a patient’s COVID-19 status.
- Patient viral load at the time of collection: The SARS-CoV-2 virus also has a part to play in test performance. Viral load may vary over time as the infection progresses and perhaps favor one compartment in the body over another.4,7 Symptom and vaccination status may also influence the viral load. Early COVID-19 studies suggest viral load from the NP region may vary back and forth from a high to low viral load state over the course of an intensive care unit stay. Serial swabbing of patients may also impact detection. Collection intervals of <24 hours may result in false negative results on subsequent specimens due to the virus having insufficient time to repopulate the collection site. Figure 1 shows internal data at UC Davis Health evaluating the SARS-CoV-2 viral RNA load between paired NP versus AN swabs. There is a statistically significant reduction in mean viral load for AN swabs compared to NP specimens.
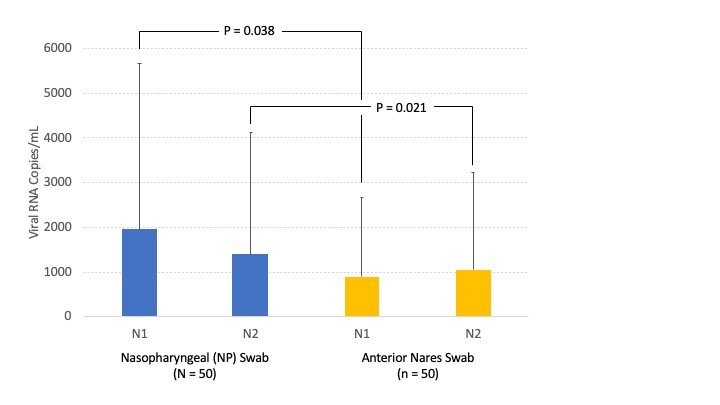
Viral Load of paired NP (blue) versus AN (yellow) swab samples based on targeting of two regions (N1 and N2) within the SARS-CoV-2 nucleocapsid gene. The test is validated for investigational use only. The limit of detection of this assay has been determined to be 600 copies/mL based on the FDA reference panel.
- Specimen matrix: Sample viscosity impacts the performance of common SARS-CoV-2 tests.5,6 For example, many PCR tests require pipetting steps which may be automated via robotics or performed manually. High viscosity could prevent accurate pipetting and compromise test performance. Viscosity issues are often observed in saliva and lower respiratory tract samples. Additives may be used to reduce viscosity but at the price of further diluting a sample and reducing the viral load for testing.
- Presence of interfering or diluting substances: Patients may use nasal medications or other compounds which may directly interfere with SARS-CoV-2 assays.6 These compounds may also potentially dilute the sample to reduce viral load below an assay’s detection limit.
- Specimen transportation conditions: Samples should be transported correct media which contains compounds to limit degradation of viral nucleic acids and inhibit growth of contaminating bacteria.4,6 Transport on ice is also recommended to further minimize specimen degradation. Delays in transportation may, again, impact specimen quality prior to testing.
SUMMARY
At UC Davis Health, NP and AN swab samples are acceptable specimen types for SARS-CoV-2 RNA testing by PCR across all platforms. The AN swab is less invasive for patients but may exhibit lower sensitivity compared to the NP swab. Many other factors influence SARS-CoV-2 test sensitivity including sample quality, presence of interfering substances, and delayed/inappropriate transport conditions. It is important for healthcare providers to be aware of these factors and weigh the pros and cons between NP versus AN swabs.
REFERENCES
- IDSA Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing (Published 12/23/2020): Accessed on August 24, 2021.
- National Public Radio website: Accessed on August 24, 2021.
- Zhou Y, O’Leary TJ. Relative sensitivity of anterior nares and nasopharyngeal swabs for initial detection of SARS-CoV-2 in ambulatory patients: Rapid review and meta-analysis. PLOS One 2021.
- Callahan C, Lee RA, Lee GR, et al. Nasal swab performance by collection timing, procedure, and method of transport for patients with SAR-CoV-2. J Clin Microbiol 2021;59:e00569-21.
- Landry ML, Criscuolo J, Peaper DR. Challenges in the use of saliva for detection of SARS-CoV-2 RNA in symptomatic outpatients. J Clin Virol 2020;130:104567.
- Vandenberg O, Martiny D, Rochas O, et al. Considerations for diagnostic COVID-19 tests. Nat Rev Microbiol 2021;19:171-183.
- Lim AY, Cheong HK, Oh YJ, et al. Modeling the early temporal dynamics of viral load in respiratory tract specimens of COVID-19 patients in Incheon, the Republic of Korea. Int J Infect Dis 2021;108:428-434.



