Jasper Zheng, M.D. and Anna Maria Romanelli, Ph.D.
The numbers of primary total hip and knee arthroplasties have been increasing over time, with 332,000 total hip and 719,000 total knee arthroplasties performed in 2010 in the United States. It is estimated that 3,481,000 primary total knee and 572,000 primary total hip arthroplasties will be performed annually in the US by 2030. With the increase in prosthetic joint implantations, serious complications of prosthetic joint infections (PJI) of the hip and knee is also on the rise.
The diagnosis of prosthetic joint infections (PJI) poses a significant challenge since real evidence-based guidelines to aid clinicians in choosing the most accurate diagnostic strategy are lacking. Despite a significant amount of basic and clinical research in this field, many questions pertaining to the definition of infection as well as diagnosis and management of these infections remains unanswered. Clinical practice guidelines for the diagnosis and management of PJI have been proposed by a number of organizations, including the 2021 European Bone and Joint Infection Society (EBJIS) criteria, the 2018 International Consensus Meeting (ICM) criteria, the 2013 ICM criteria, the 2013 Infectious Disease Society of America guidelines (IDSA), and the 2011 Infection Society (MSIS) criteria. These guidelines stress the importance of using a multi-disciplinary approach to aid in the diagnosis of PJI, requiring supporting evidence from clinical examination, laboratory results, microbiological culture identification, histological interpretation, and intraoperative findings. These guidelines are meant to serve as an educational tool designed to assist practitioners in providing appropriate care for patients. It is anticipated that consideration of these guidelines may help reduce morbidity, mortality and the costs associated with PJI.
In this Lab Best Practice Blog, we will review the criteria used to establish infection and explore the process of diagnosing prosthetic joint infection.
Risk Factors of Prosthetic Joint Infection
Patient-related risk factors for prosthetic joint infection include prior revision arthroplasty or prior same site prosthetic joint infection, tobacco use, obesity, rheumatoid arthritis, malignancy, immunosuppression and diabetes mellitus.
Postoperative risk factors include wound healing complications (such as superficial infection, hematoma, delayed wound healing, wound necrosis, or dehiscence), atrial fibrillation, myocardial infarction, urinary tract infection, prolonged hospital stay, and at any time postoperatively, Staphylococcus aureus bacteremia. It is important to consider all of these potential factors when assessing for risks of postoperative PJI.
Categorization of Prosthetic Joint Infection
The classification scheme useful for identification of PJI is simply based on the time to infection, classified as early, delayed, or late onset. Early onset PJI occurs less than three months after the last surgery. These infections are most commonly initiated at the time of operation, through intraoperative contamination, and are usually caused by relatively virulent microorganisms. Delayed onset PJI occurs after 3 months but before 12 or 24 months. Different authors have used different time points to differentiate between delayed and late onset PJIs. However, regardless of the cutoff used, the common theme is that these infections are also typically acquired at the time of surgery but are caused by less virulent microorganisms such that the overt presentation of infections does not occur within the first 3 months. Late onset PJI, usually occurs 12-24 months after surgery and is likely due to a hematogenous infection but may also be due to extremely indolent infection initiated at the time of surgery.
Pathogens involved in Prosthetic joint infection
Timing of infection can serve as a clue to the pathogen identity. It is important to keep in mind that some pathogens present a significantly higher risk than others.
- Early onset (less than three months after surgery):
- Staphylococcus aureus
- Anaerobes
- Polymicrobial infection
- Delayed onset (three to twelve months after surgery):
- Coagulase-negative staphylococci
- Cutibacterium (Propionibacterium) species
- Enterococci spp.
- Late onset (greater than twelve months after surgery)
- Staphylococcus aureus
- Gram-negative bacilli
- Beta-hemolytic streptococci
Pathogenesis
The pathogenesis of prosthetic joint infections by pathogens is dependent on the formation of biofilm. Biofilms are complex communities of microorganisms embedded in an extracellular matrix that forms on surfaces. Pathogens will adhere to orthopedic hardware, and proliferate with elaboration of exopolysaccharides known as glycocalyx, which after a certain period, will coalesce into biofilm. This biofilm microenvironment serves as a barrier that renders host defenses and antimicrobials less effective. Complicating matters further, pathogens hidden deep in the biofilm have characteristic indolent low metabolic rate which also prevent accurate culture identification. Together, S. epidermis, S. aureus and Pseudomonas aeruginosa make up almost 75% of the biofilms found in medical devices. S. aureus and Staphylococcus epidermis are the most common biofilm-forming bacteria.
Diagnosis of prosthetic Joint Infection
As mentioned previously, there are a number of criteria published, but given its simplicity and wide use, the Musculoskeletal Infection Society (MSIS) 2011 diagnostic criteria appear to be favored by the orthopedic specialty to help establish PJI.
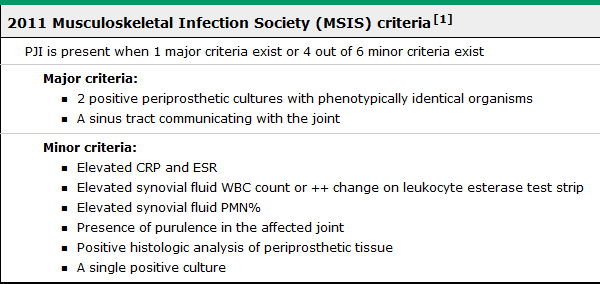
Preoperative Evaluation of Suspected Prosthetic Joint Infection:
- Clinical manifestation
- Patient with joint prosthesis and symptoms of infection
- Joint pain, warmth, erythema, induration, incision site edema, sinus tract, persistent wound drainage, wound dehiscence, joint effusion, and/or fever
- Patient with joint prosthesis and symptoms of infection
Then
- Plain radiograph
- should be performed in all patients with a suspected prosthetic joint infection
- Radiographic findings suggesting possibility of PJI:
- abnormal lucency larger than 2 mm in width at the bone-cement interface
- changes in the position of prosthetic components
- cement fractures
- periosteal reaction
- motion of components on stress views
- leukocyte scans, positron emission tomography (PET) scans, computed tomography (CT) scans, magnetic resonance imaging (MRI) scans, or bone scans are not useful for routine diagnostic evaluation in most cases of suspected PJI
- Sedimentation rate or C-reactive protein (CRP) tests
- When suspected prosthetic joint infection is not clinically apparent, Sedimentation rate or C-reactive protein (CRP) tests should be performed.
- Timing dependent
- early-onset PJI
- ESR is not useful
- CRP is often >100 mg/L
- delayed and late onset PJI
- ESR is often >30 mm/hour
- CRP is often >10 mg/L
- if both ESR and CRP are negative, the likelihood of PJI is low.
- PJI may be present in the setting of normal ESR and CRP
- Possible late PJI
- infection due to pathogens of low virulence
- prior antibiotic use
- immunosuppression
- early-onset PJI
Warning:
- Following joint replacement surgery, the CRP may require two to three weeks to return to normal preoperative values, and the ESR may require up to a year to return to normal preoperative values.
Interpret with caution in the setting of coexistent chronic inflammatory disease, which can also elevate serum inflammatory markers.
Followed by
- Arthrocentesis:
- Arthrocentesis is performed for all patients with suspected acute prosthetic joint infection.
- Unless the diagnosis is clinically evident, surgery is planned, and antimicrobials can be safely withheld prior to surgery.
- May not be necessary if surgery is planned and the result is not expected to alter management.
- Arthrocentesis is advised in patients with a chronic painful prosthesis with unexplained elevated sedimentation rate or CRP level.
- If the patient is medically stable, withhold antimicrobial therapy for at least 2 weeks prior to collection of synovial fluid for culture will increase the likelihood of recovering an organism.
- Synovial fluid may be sent for culture in a sterile tube (ideally a red-top tube with no additives and a tube with an anticoagulant such as ethylenediaminetetraacetic acid to guard against clotting) or in blood culture bottles. If blood culture bottles are used, synovial fluid also should also be sent in a sterile container for Gram stain. Use of blood culture bottles may increase the likelihood of recovering nonpathogenic skin contaminants; in such cases, culture results should be interpreted in the context of the Gram stain result.
- Arthrocentesis is performed for all patients with suspected acute prosthetic joint infection.
- Interpretation of synovial fluid analysis
- Time of onset dependent
- early-onset PJI : synovial fluid cell count is often >10,000 cells/microL (>90 percent neutrophils)
- delayed- and late-onset PJI : the synovial fluid cell count is often >3000 cells/microL (80 percent neutrophils)
- The most accurate cell count threshold for knee PJI:
- 1630 cells/microL (sensitivity and specificity 84 and 82 percent, respectively)
- with 60 percent neutrophils (sensitivity and specificity 80 and 77 percent, respectively) [73].
- The most accurate cell count threshold for hip PJI:
- 2582 cells/microL (sensitivity and specificity 80 and 85 percent, respectively)
- with 66 percent neutrophils (sensitivity and specificity 82 percent).
- Use of saline irrigation to obtain synovial fluid may confound the cell count.
- Time of onset dependent
Figure 1. Summary of PJI evaluation.
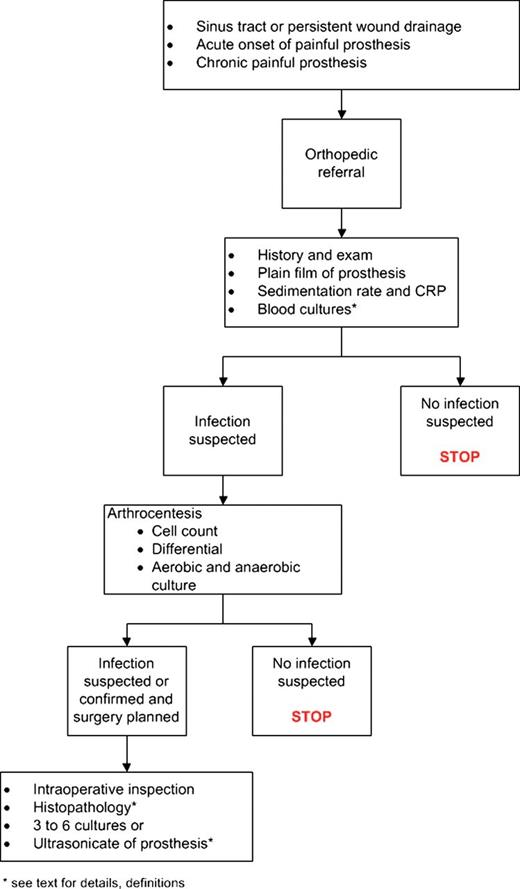
Intraoperative evaluation
- Histopathological Assessment and Interpretation During Frozen Section
- Presence of polymorphonuclear cells is indicative of an acute inflammatory reaction supporting the diagnosis of PJI
Diagnosis requires a threshold of at least five PMNs per HPF (sensitivity >80%, specificity >90 percent) in periprosthetic tissue.
Evaluation of Suspected Prosthetic Joint Infection Culture
- When to send culture:
- In patients with a sinus tract, drainage (collected by aspiration) should be sent for culture; swabs from the sinus tract should not be sent given discordance with deep cultures.
- In patients with fever or other systemic manifestations of infection, blood cultures (two sets) should be obtained.
- Cultures of a superficial wound or sinus tract are often positive because of microbial colonization from the surrounding skin and should therefore be avoided.
- Culture of prosthesis
- If the prosthesis is removed, the implant or its components can be cultured in enrichment broth. However, the risk of contamination during specimen processing is high.
- Cultures of periprosthetic tissue provide the most reliable means of detecting a pathogen
- - sensitivity of these cultures ranges from 65 to 94 percent
- At least three to six intraoperative tissue specimens should be sampled for culture.
- Diagnosis of PJI established from culture
- may be established in the setting of two or more periprosthetic cultures with phenotypically identical organisms (a combination of preoperative synovial fluid aspiration culture and intraoperative tissue culture or ≥2 intraoperative tissue cultures)
- Growth of a virulent microorganism (eg, S. aureus) in a single specimen of a tissue biopsy or synovial fluid may also represent PJI.
- Cultures are negative for 7-39% of patients with suspected prosthetic joint infection.
- Cultures are more likely to be positive for early-onset prosthetic joint infection.
- Cultures are more likely to be negative if insufficient tissue was sent or if only swabs were collected.
- Culture yields are more likely to be diminished and negative if antibiotics were administered prior to culture collection.
- Negative cultures can be attributed to slow growing and difficult to detect variants of staphylococci, or fastidious pathogens that includes Coxiella Burnetii, Brucellosis, Bartonellosis, Abiotrophia defectiva, Granulicatella adiacens, Mycoplasma, Mycobacteria, and Fungi
- Cultures may be negative due to prolonged transport time to the microbiology laboratory.
- Swab cultures have a low sensitivity and should be avoided.
- To detect cases of low-grade infection, antimicrobial therapy should be discontinued at least two weeks before tissue specimens are obtained.
- If revision surgery is planned, perioperative prophylaxis should not be administered until after tissue specimens have been collected for culture.
Summary
The diagnosis of prosthetic joint infection involves first establishing whether the joint is infected and then, if it is, defining the involved microorganism(s). The goal of treatment is to cure infection, prevent recurrence, and achieve a pain-free, functional joint. This can best be achieved by a multidisciplinary team, including an orthopedic surgeon, clinical microbiologist, and infectious diseases specialist. Antimicrobial agents alone, without surgical intervention, ultimately usually fail. The quality of surgical debridement is critical. Prosthetic joint infection (PJI) is a serious complication of prosthetic joint implantation. The epidemiology, microbiology, clinical manifestations, and diagnosis described here all serve as an important piece of the puzzle required to provide effective prevention, management, and treatment of prosthetic joint infection.
Resources:
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645-1654. doi:10.1056/NEJMra040181
- Parvizi J, Tan TL, Goswami K, et al. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty. 2018;33(5):1309-1314.e2. doi:10.1016/j.arth.2018.02.078
- Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
- Tomas X, Bori G, Garcia S, et al. Accuracy of CT-guided joint aspiration in patients with suspected infection status post-total hip arthroplasty. Skeletal Radiol. 2011;40(1):57-64. doi:10.1007/s00256-010-0940-2
- Berbari, E. et al. Prosthetic joint infection: Epidemiology, microbiology, clinical manifestations, and diagnosis. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on 07/05/2021.)
- Douglas R. Osmon, Elie F. Berbari, Anthony R. Berendt, Daniel Lew, Werner Zimmerli, James M. Steckelberg, Nalini Rao, Arlen Hanssen, Walter R. Wilson, Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America, Clinical Infectious Diseases, Volume 56, Issue 1, 1 January 2013, Pages e1–e25, https://doi.org/10.1093/cid/cis803
- Jämsen E, Huhtala H, Puolakka T, Moilanen, Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. T J Bone Joint Surg Am. 2009 Jan; 91(1):38-47.
- Peersman G, Laskin R, Davis J, Peterson M., Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001 Nov; (392):15-23.



