Elham Kamangar, MD, Surgical Pathology Fellow (PGY6)
John Bishop, MD, Health Sciences Clinical Professor, Vice Chair for Clinical Services, Department of Pathology and Laboratory Medicine
Background: In the era of precision medicine, human epidermal growth factor receptor 2 (HER2) is one of the important predictive and prognostic biomarkers in breast cancer. The HER2 status of a patient's tumor can be analyzed at the protein level by immunohistochemistry (IHC) and at the chromosome level by in situ hybridization (ISH) techniques. Accurate determination of HER2 status is critical for optimizing breast cancer outcomes. In 2007, the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) developed guidelines for HER2 testing to reduce inaccuracy. These guidelines are revised in 2013 and 2018,[1] to further optimize HER2 testing and include emerging techniques.
Current guidelines for test performance: Formalin fixed, paraffin embedded tumor tissue samples are appropriate for assay. Ideally, buffered formalin should be used for fixation because the use of alternative fixatives such as Bouin's fixative will preclude testing by fluorescence in situ based methods. Other methods of tissue fixation can also adversely affect antigen reactivity.[2] It is also recommended that cold ischemia time, defined as the time from the removal of the tissue from the patient to the initiation of tissue fixation, be shortened as much as possible, specifically, no more than 1h and the required fixation time is 6-72 hours (as is the current practice in UC Davis pathology laboratory).
Laboratories providing a testing service should be carrying out a minimum of 250 assays each year for immunohistochemical detection of HER2. This target value has been set to ensure continuing expertise of assay providers. There is evidence of higher consistency of assay quality when tests are performed by high volume reference laboratories. Specifically, laboratories with low volumes using immunohistochemistry (IHC) have higher frequencies of IHC positive cases and reduced levels of IHC/FISH (fluorescence in situ hybridization) concordance, which is related to a higher proportion of IHC positive/FISH negative results. For both IHC and FISH based HER2 testing, comprehensive standardization of methodology, including monitoring of scoring procedures, and the inclusion of validated controls, is mandatory. The inclusion of controls and their detailed scrutiny are essential to ensure test accuracy. A recommended positive control or controls producing results close to important decision making points and a negative control are recommended. For the assessment of both IHC and FISH preparations training and experience in the interpretation of histological characteristics of breast tissue are essential. The recognition of different histological tumor types is required. In particular, the HER2 status should be determined only on the invasive portion of the tumor and IHC positivity or FISH amplification should not be reported as a positive result in isolation.
Scoring IHC:
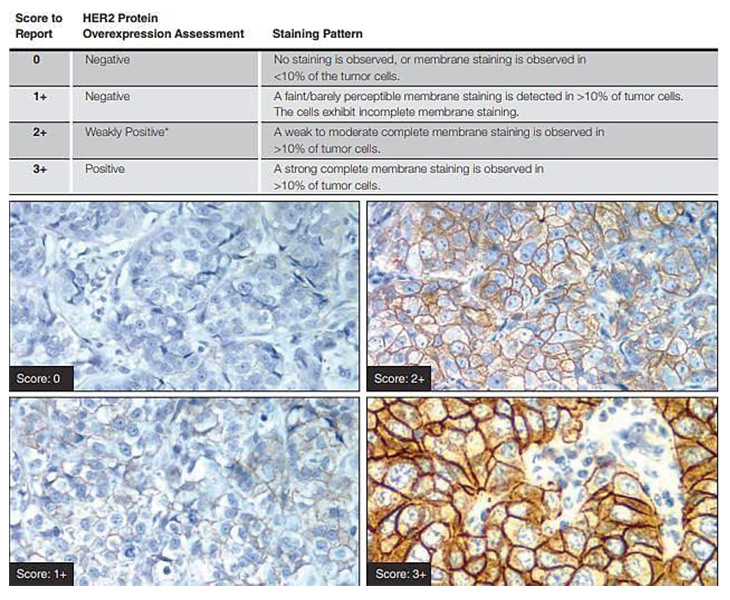
Only membrane staining of the invasive tumor should be considered when scoring IHC tests. If a commercial kit assay system is used, it is recommended that laboratories adhere strictly to the kit assay protocol and scoring methodology (DAKO Hercep kit is being used in our laboratory). Local modifications of techniques can lead to false positive and negative assay results. The scoring method recommended is a semi-quantitative system based on the intensity of the reaction product and the percentage of membrane positive cells, giving a score range of 0 to 3+ Samples scoring 3+ are regarded as unequivocally positive and 0/1+ as negative. Equivocal 2+ samples require confirmation using another analysis system, ideally FISH. Observers should be aware of the range of common artefacts, including edge artefacts, which can be problematic in small biopsy samples, and the effects of variation in method sensitivity, such as excessive antigen retrieval, leading to background staining and normal cell membrane reactivity.
The matter of post-analytical phase: Intra-tumoral heterogeneity: A real challenge and widely discussed matter of debate is represented by intra-tumoral HER2 heterogeneity. If on one side decisions for therapy require a yes/no answer, in the other HER2 status derives from a continuum of gene copy number and protein expression.[3] About heterogeneity a distinction should be made. The first kind is represented by presence of two distinct populations of cells (i.e., two different clones of cancer cells within a lesion), one completely negative for HER2 and the other clearly positive (HER2 amplified). The second kind of heterogeneity, which leads to considerable troubles in FISH reporting, is represented by those tumors in which scattered HER2 amplified cells are identified within a homogeneous background of cells that substantially lack HER2 gain or amplification. In terms of biology of HER2+ tumors a “brand new” topic is the recent demonstration of another biological mechanism underpinning HER2 activation, i.e., activating mutations. Recent next generation sequencing studies have brought to the front-line the presence of HER2 mutations in breast cancer, a phenomenon known since 2005 and that has been neglected due to the most pervasive mechanism of HER2 gene amplification.[4] Data from eight breast cancer genome-sequencing projects have identified 25 patients with HER2 somatic mutations who developed cancers lacking HER2 gene amplification.[5]
In the meantime while we wait for these critical preclinical data to pave the way to HER2 sequencing-directed breast cancer clinical trials, the next challenge for pathologists will be to delineate the characterization of HER2 mutation carriers. From data reported by Bose et al., putative candidates seem to be identified with ER positive breast carcinomas (both ductal and lobular carcinomas analyzed), either HER2 negative (score 0/1+) or showing equivocal HER2 expression (score 2+).[5]
References:
- Antonio C. Wolff, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. J Clin Oncol. 2018 Jul 10;36(20):2105-2122.
- Ellis IO, Bartlett J, Dowsett M, Humphreys S, Jasani B, Miller K, Pinder SE, Rhodes A, Walker R. Best Practice No 176: Updated recommendations for HER2 testing in the UK. J Clin Pathol 2004 Mar; 57(3): 233-7.
- Oakman C., Sapino A., Marchio C., Pestrin M., Biganzoli L., Di Leo A. (2010). Chemotherapy with or without trastuzumab. Ann Oncol. 2010 Oct; 21 Suppl 7():vii112-9.
- Weigelt B, Reis-Filho JS. Activating mutations in HER2: neu opportunities and neu challenges. Cancer Discov. 2013 Feb; 3(2):145-7.
- Bose R, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer.Cancer Discov. 2013 Feb; 3(2):224-37.



