Clayton LaValley, MD; John Paul Graff, DO
Introduction: Reference Intervals and their Importance
The Centers for Medicare & Medicaid Services, HHS stipulate that a written procedure manual be available, and followed, by all laboratory personal. One key aspect of these standardized procedure manuals are test specific reference intervals. Reference intervals (RIs), or reference ranges, are typically defined as the central 95% of laboratory results expected in a healthy reference population. A patient’s laboratory test results are compared to these established healthy or “normal” RIs for the purpose of decision marking. The importance of establishing accurate and representative RIs cannot be understated as nearly 80% of medical decisions are based on the interpretation of numerical laboratory reports1. This interpretation can lead to a change in therapeutic management, help reach a diagnostic threshold, or give insight into the patient’s underlying physiologic state. As such, the quality of the RIs may play as large of a role in result interpretation as the quality of the result itself.
Given its importance, Clinical and Laboratory Standards Institute (CLSI) published guidelines for defining, establishing, and verifying RIs in the clinical laboratory in 20102. These guidelines are recognized by the FDA3 and are referenced by the College of American Pathologists (CAP) in their accreditation checklists4. In addition, the CAP requires that all patient laboratory results be reported with a RI, while the International Organization for Standardization (ISO) 15189 standard for laboratory accreditation recommends the periodic review of RIs to ensure continued applicability5.
The establishment of de novo RIs can be challenging and costly for clinical laboratories as it requires labs to perform a RI study following published guidelines. These studies include following a robust and well-defined protocol with a statistically sufficient group of healthy reference subjects (minimum 120). The process can be quite strenuous, with some of the recommended study elements including in depth descriptions of analyte characteristics, method analysis, reference population demographics, reference population medical history, data interpretation, statistical analysis, and the clinical relevance of the RI1-3,6. Practically, it is not feasible for a single laboratory to perform a study for each analyte at a high enough standard to ensure continued high quality patient care. For this reason, most laboratories use previously established RIs for clinical use in a process known as transference. The most commonly used external sources include manufacturers’ package inserts, textbooks, multicenter studies, publications, national or international expert panel recommendations, guidelines, and other laboratories7.
CLSI Guidelines: Transference and Verifying RIs
As it is the responsibility of individual laboratories to use RIs that are appropriate for their patient population and methodologies, clinical laboratories may transfer in and verify RIs from any of the external sources listed above. This is done assuming that the original RI study from the external source was done with robust methodology, and statistical procedures. The two main scenarios for which RIs are transferred and verified are:
- When reference values originate from a different population and different laboratory method than the clinical laboratory where it is being implemented.
- When reference values originate from a laboratory that shares methodologies and has a similar population as the clinical laboratory where it is being implemented.
The processes for each scenario are similar, however, in the first scenario, a method comparison study should be performed with values spanning the RI. The comparison should then be statistically analyzed to determine the characteristics of the best-fit regression line with the values used to transfer the RI8. The best-fit regression line should have a slope bias close to 1, a y-intercept close to 0 and a correlation coefficient close to 1. In this sense, the method comparison acts as a screening test to ensure if the RI is appropriate to undergo transfer.
After a RI has been transferred, it must be verified. By CLSI, there are 3 approved approaches to verifying RIs:
- Subjective assessment
- Comparing to a small number of reference individuals (n=20)
- Comparing to a large number of reference individuals (n=60 to 120)
Though option 3 has a smaller sample size than a full RI study, it is still the least preferred method for verification as it is still more prohibitive in terms of time/resource requirements than option 2. As such, the standard approach for verification in routine practice is to collect and analyze 20 samples (per sex and/or age group) from healthy subjects within the laboratory’s population. Statistical approaches (e.g. Reed, Dixon, or Tukey methods) should be used to identify and remove outliers, while subsequently replacing those removed with new samples9-11. Of those 20 samples, if no more than 2 of the 20 samples fall outside of the RI, then the RI is valid and can be verified. If 3 to 4 of the 20 samples fall outside of the RI, then a second set of 20 samples must be obtained (using the same process as above). If again, 3 or more of the new samples fall outside of the RI, then the RI is not valid and cannot be verified. In this instance, it is not recommended to use the RI on the analytical platform under investigation. This is also the case if the initial study has 5 or more of the 20 samples fall outside of the RI2,7. The process for verification is summarized in Figure 1.
Figure 1: CLSI process for the verification of outside reference intervals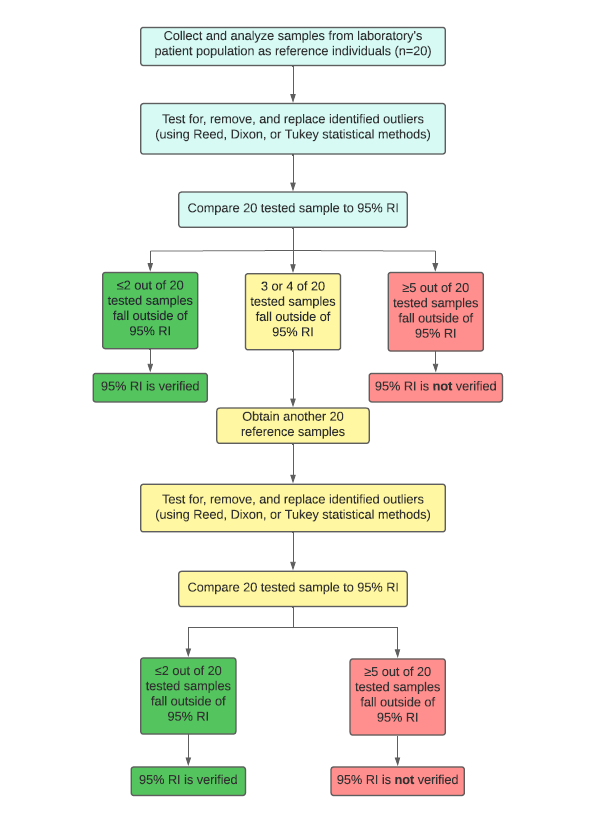
References
- Katayev, Alex et al. “Establishing reference intervals for clinical laboratory test results: is there a better way?” American journal of clinical pathology vol. 133,2 (2010): 180-6. doi:10.1309/AJCPN5BMTSF1CDYP
- Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved Guideline – Third Edition. CLSI EPC28-A3c. Wayne (PA): Clinical and Laboratory Standards Institute, 2010.
- FDA U.S. Food & Drug Administration. “Recognized Consensus Standards: CLSI EP28-A3c.” FDA. Accessed March 30 2022.
- College of American Pathologists. “Accreditation Checklists.” CAP. Accessed March 30 2022.
- International Organization for Standardization. ISO 15189:2012 (E) Medical laboratories – Requirements for quality and competence, Third edition, 2012-11-01.
- Jones, Graham, and Antony Barker. “Reference intervals.” The Clinical biochemist. Reviews vol. 29 Suppl 1,Suppl 1 (2008): S93-7.
- Ozarda, Yesim, Higgins, Victoria and Adeli, Khosrow. "Verification of reference intervals in routine clinical laboratories: practical challenges and recommendations" Clinical Chemistry and Laboratory Medicine (CCLM), vol. 57, no. 1, 2019, pp. 30-37.
- Method procedure comparison and bias estimation using patient samples, approved guideline, 3rd ed. Wayne (PA): CLSI, 2013. CLSI document EP09-A3.
- Dixon WJ. Processing data for outliers. Biometrics 1953;9:74–89.2307/3001634
- Reed AH, Henry RJ, Mason WB. Influence of statistical method used on the resulting estimate of normal range. Clin Chem 1971;17:275–84.1093/clinchem/17.4.275
- Tukey JW. Exploratory data analysis. Boston (MA): Addison-Wesley, Reading (PA), 1977:1–688.



