CTSA Trial Innovation Network
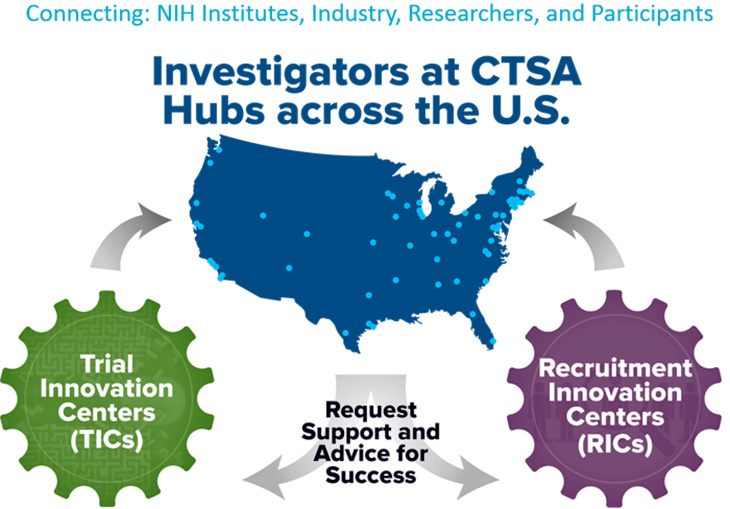
The Trial Innovation Network (TIN) is a resource that provides operational innovation for multicenter clinical trials by leveraging the expertise of the Clinical and Translational Science Awards (CTSA) network to assist investigators with planning and execution.
Key elements of the TIN include:
- Central IRB support
- Standardized contracts
- Data-driven recruitment strategies and stakeholder engagement
- Assistance in protocol design and trial efficiency
- Harmonized IT tools and approaches
The TIN consists of three key organizational partners:
- CTSA Program Hubs (e.g. UC Davis Clinical and Translational Science Center)
- Innovate processes to increase the quality and efficiency of translational research, particularly of multisite trials.
- Encourage faculty and investigators at their institutions to generate ideas for trials and studies.
- Recognize the essential contributions and efforts of their local teams in executing multi-center clinical trials.
- Create a culture in which key stakeholders play unique and important roles, and ultimately work together to build a national system to conduct clinical trials better, faster, and more cost-effectively.
- Trial Innovation Centers (TICs)
- Coordinate and provide innovative, high quality operational support for clinical trials.
- Focus on operational excellence, operational innovation, and quality by design.
- Recruitment Innovation Center (RIC)
- Provide tools and services to enhance participant recruitment and retention.
- Focus on innovative and evidence-based approaches to participant recruitment, retention, and engagement.
Resources
How to start?
Prior to submitting a proposal to the TIN, contact the UC Davis CTSC Hub Liaison Team and submit a service request.
