Two decades in the making: Scientists discover molecule that strengthens bones
The finding could help treat bone-weakening conditions like osteoporosis
Researchers at UC Davis Health and UC San Francisco have identified a molecule known as Maternal Brain Hormone (CCN3) that increases bone density and strength.
Their study, recently published in Nature, explains how women’s bones remain strong during breastfeeding, despite calcium being stripped from bones to support milk production.
The new discovery has the potential to treat a variety of bone conditions, including osteoporosis.
UC Davis played a major role leading to the breakthrough
The foundation for the study began at the UC Davis Department of Rheumatology nearly two decades earlier.
“Interestingly, this all started with mice we got from Kamala Harris’ mother’s lab at the Lawrence Berkeley National Laboratory,” quipped Nancy E. Lane, distinguished professor of rheumatology at UC Davis Health and co-author of the study. “We started to see that in progesterone receptor knock out female mice had both more bone and stronger bone than normal female mice.”
(Vice President Harris’ mother was a scientist in the East Bay).
Lane and Wei Yao, professor of internal medicine at UC Davis Health, studied the impact of nuclear progesterone receptors on bones. These receptors are proteins that bind to the hormone progesterone.
Their study evaluated if a peak bone mass could be achieved in a mouse model with no nuclear progesterone receptor or when using a drug to block the work of these receptors.
“We noticed that the female mice experienced increased bone formation when we removed the nuclear progesterone receptor from the skeletal stem cells during their rapid bone growth period,” explained Lane. “Our research ended up going in a different direction, but this initial work suggested a crucial role for progesterone signaling in bone acquisition.”
Years later, Lane received a call from the Ingraham Lab at UC San Francisco.
“They reached out asking if we were the people who had researched progesterone signaling in bone acquisition,” recalled Lane. “They shared about their work on blocking a particular estrogen receptor found in select neurons in a small area of the brain that led to huge increases in peak bone mass in female mice, but not so much in male mice. Since the group was made up of molecular endocrinologists, they reached out to a bone research group and that was when we started working together, which led us to this discovery.”
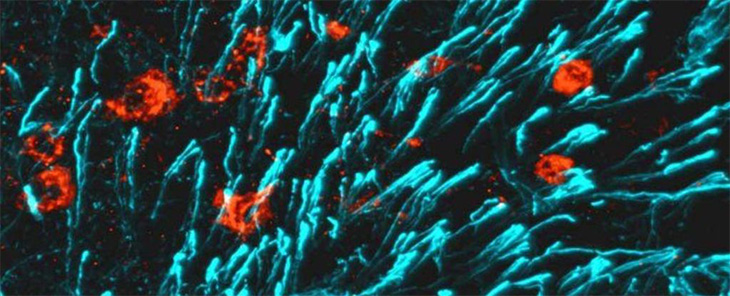
Discovering CCN3 role in bone growth
The multidisciplinary team of scientists set out to determine why blocking a particular estrogen receptor resulted in female mice with particularly high bone density.
“We knew that the estrogen receptor was present in the arcuate nucleus of the hypothalamus — the structure of the brain that is involved with nutrient sensing,” said Lane. “Therefore, the researchers examined how removing the ER receptor from the ARC and feeding the mice a high fat diet would affect bone density and the hormones being produced.”
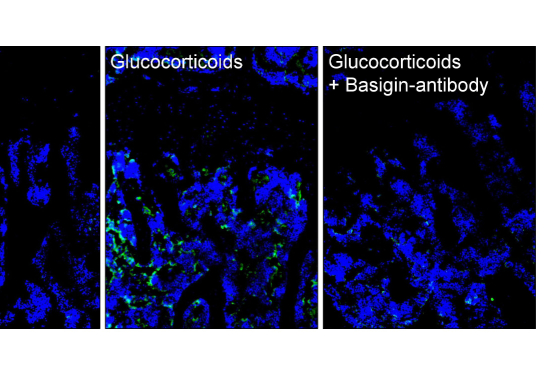
The team found that the mice placed on a high-fat diet reverted to having a normal bone density. An analysis of which bone-strengthening factors decreased led the team to pinpoint CCN3 as the factor responsible in the increases in bone density. CCN3 is a hormone linked to many biological processes, including cell growth and migration.
Next, the team added CCN3 to mouse skeletal stem cells in a petri dish — and discovered that when treated with CCN3, bits of bone formed. The team also tested CCN3 in elderly mice with bone fractures, which typically do not heal well. When they applied a hydrogel patch containing CCN3 onto a fractured bone, it promoted bone growth at the site of the fracture.
“This is incredibly exciting because we have therapies to prevent bone loss, but we are still limited in terms of the drugs that stimulate bone formation,” explained Lane. “Discovering this molecule in which the activity is through the skeletal stem cell could help us create future therapies to potentially increase bone regeneration to treat osteoporosis, and many other health conditions in which more osteoblasts are needed.”
Discovering this molecule in which the activity is through the skeletal stem cell could help us create future therapies to potentially increase bone regeneration to treat osteoporosis, and many other health conditions in which more osteoblasts are needed.” —Nancy E. Lane
What’s next?
The team of researchers now plan to carry out future studies on the molecular mechanisms of CCN3, as well as the potential of the molecule to treat a variety of bone conditions.
Lane with her colleagues in Orthopaedics at UC Davis Health, Thomas H. Ambrosi, assistant professor of musculoskeletal disorders, and Kent Leach, professor of orthopedic surgery, also want to learn whether CCN3 can help regenerate cartilage.
Osteoarthritis, the most common form of arthritis, affects millions of people worldwide. The condition occurs when the cartilage and other tissues within the joint break down or deteriorate.
“We want to see if pumping CCN3 into the damaged cartilage of mice when there’s no oxygen or blood supply will help regenerate it,” said Lane. “Our hope is this could help us learn if CCN3 has the potential to not only treat bone-weakening conditions like osteoporosis in humans, but also other degenerative diseases.”