Andy D Jones, M.D., Pathology Resident
Sarah Barnhard, M.D., Assistant Professor and Associate Director, Transfusion Services
Grace Monis, M.D., Ph.D., Assistant Professor, Transfusion Services
Background
Recent developments in the field of coagulation testing have shown that the test previously used to monitor therapeutic dosing of unfractionated heparin (UFH) in hospitalized patients has serious drawbacks. Developments in laboratory testing, reduced cost of reagents, and comparative studies have suggested superiority in monitoring UFH with Antifactor Xa levels as compared to the more traditional activated partial thromboplastin time (aPTT).
Mechanism of action of UFH
Heparin is a naturally-occurring glycosaminoglycan polymer that has a physiologic anti-coagulant function.1 It exists as polymers of varying sizes (20,000 – 50,000 kDa) naturally, and as manufactured unfractionated heparin. (Fractionation produces concentrations of heparin molecules of similar sizes, as in low molecular weight heparin.2)
As an indirect thrombin inhibitor, the eccentric pentasaccharide sequence of the heparin molecule binds to anti-thrombin, causing a conformational change. This change increases the activity of antithrombin (ATIII), which has effects on both activated thrombin (Factor IIa) and Factor Xa. The net result is an inhibition of the coagulation cascade (leading to anti-coagulation).1
While heparin is a naturally occurring molecule, unfractionated heparin is most commonly manufactured from the mucosal tissues of slaughtered pigs and cows (gut and lung mucosa, respectively). It has a short half-life (~ 2 hours), is low cost, has non-renal elimination, and is readily reversible in the inpatient setting.3
Historical measurement of UFH activity
Traditional laboratory measurement of heparin activity was accomplished via the Activated Partial Thromboplastin Time (aPTT) test. The test involves obtaining a patient’s plasma (in a negatively-charged centrifuged sodium-citrate tube). The citrate reversibly chelates the available calcium, leading to inhibition of activation of the coagulation proteins (i.e. ensuring the sample doesn't irreversibly clot before arriving at the lab). Excess calcium is then added to the tube, and the clotting time measured.1 This provides an estimate of the functional performance of the intrinsic pathway. Typical estimates of appropriate anticoagulation (depending on patient populations) range from 1.5 – 2.5 fold increase in clotting time over the patient’s baseline.2
Problems with aPTT in UFH monitoring
While the aPTT assay has long been used in monitoring patient response to UFH, laboratorians have noticed an increasing array of complications that render the aPTT an unfavorable choice in this scenario.
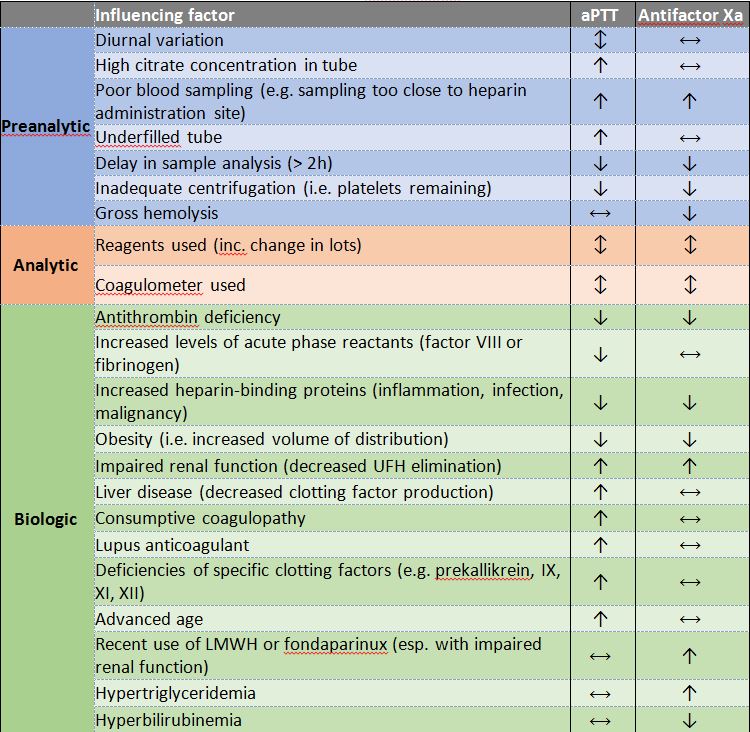
Pre-analytic variables that reduce the efficacy of aPTT include diurnal variation coagulation factors, which can result in a spuriously low aPTT (morning sampling) or high aPTT (evening sampling); variations in the concentration of citrate in the collection tube, which can result in a spuriously high aPTT; and underfilled sample tubes, which can result in a spuriously high aPTT.3
Analytic variables have also been shown to have a significant impact on aPTT monitoring of UFH therapy, including the reagents used (including variations from lot to lot) and the instrument used. While there has been a desire to standardize aPTT values across institutions (as has been done for the INR), the significant variability has precluded this possibility.3
Finally, biologic variables also contribute to the problems with aPTT, including variations in levels of acute phase reactants in critically ill patients (e.g., Factor VIII or fibrinogen), which can cause spuriously low aPTT values; liver disease or other deficiencies of clotting factors, causing a spuriously high aPTT; consumption of coagulation factors, leading to a spuriously high aPTT; presence of the lupus anticoagulant, leading to a spuriously high aPTT; and advanced age, leading to a spuriously high aPTT.3
When combined, studies have shown that less than one half of the variation in patient’s aPTT values in patients receiving UFH could be explained by changes in heparin concentrations. And, when compared across laboratories (using different testing methods and different reagents), almost 10% of aPTT results were so discordant that the same patient was reported as being below the therapeutic threshold at one laboratory, and above the therapeutic threshold at another.3
Antifactor Xa assay
To correct for these deficiencies, laboratory professional agencies initially suggested correcting aPTT values based on a second assay, called antifactor Xa.1 Rather than a global anticoagulation test (like aPTT), antifactor Xa testing looks only at the functional activity of heparin.2 Heparinized patient plasma is mixed with free excess anti-thrombin to create a heparin-anti-thrombin complex (see Figure 1). This complex then binds to Factor Xa, leaving free, excess, unbound Factor Xa. A chromogenic substrate that also binds Factor Xa is then added, and the chromogenic substrate/residual Xa complex produces a color change that can be measured by common laboratory equipment.3
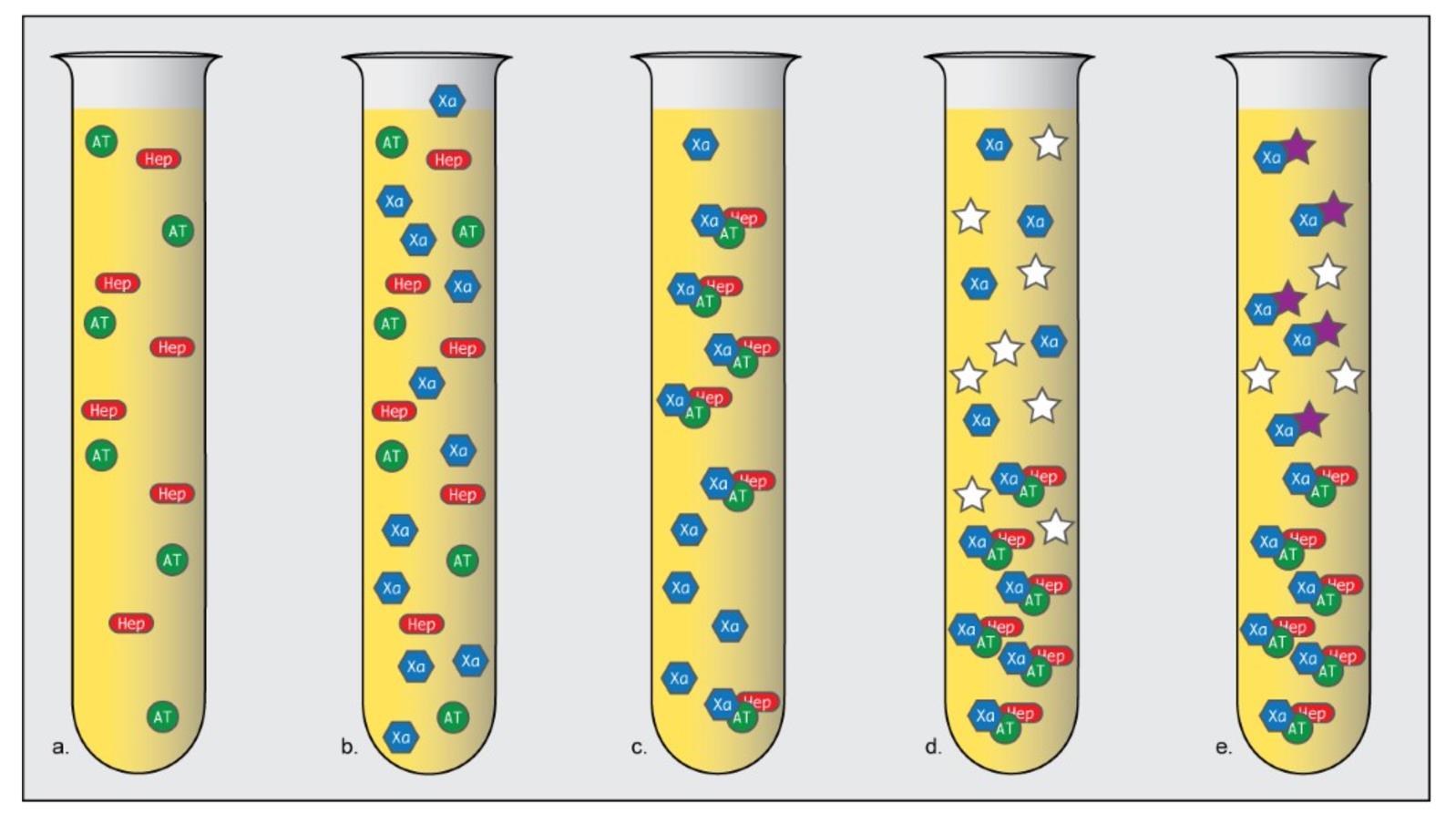
Figure 1: Overview of antifactor Xa test. A sample of heparinized patient platelet-poor plasma is obtained (a). A known amount of factor Xa is added to this sample (b) which enhances the binding of heparin and anti-thrombin (c). A chromogenic substrate is added (d) that binds to excess Xa (e), producing a color change that can be measured in a spectrophotometer. The color change is directly proportional to the amount of unbound Xa; from the degree of color change, the functional activity of heparin (inversely proportional to the color change) can be calculated. (Adapted from Mayo Medical Labs “Anti-Xa Assay for Heparin Monitoring [Hot Topic]”4.)
The suggested correction of aPTT was not adopted by many laboratories, due to the increased cost and unclear patient benefit and clinical interpretability of such an adjustment.3 However, further studies showed that this highly-specific assay, which tests only the in vitro functional status of heparin (not the entire intrinsic pathway), may have a better correlation between outcome-based anticoagulation status and antifactor Xa activity than aPTT. In clinical studies, patients managed with antifactor Xa values between 0.3-0.7 U/mL had fewer recurrent episodes of VTE and fewer bleeding episodes than did patients managed using the traditional 1.5-2.5 aPTT values, despite having received less heparin than the latter group. It's worth noting that many studies have been underpowered to achieve statistical significance when examining definite endpoints in comparing the two assays.3
Further studies have shown other benefits to using antifactor Xa monitoring, including fewer monitoring tests and dosage changes (0.65 fewer laboratory tests per patient per day; 0.85 fewer dosage adjustments per patient per day), shorter time to therapeutic dosage, higher percentage of values in dose-range, and increased inter-laboratory correlation. Cost-benefit analyses have subsequently shown that, despite a modest increase in reagent cost, overall cost per patient is equivocal or slightly less than traditional aPTT monitoring, due to reduced frequency of testing and dose-adjustment monitoring.3
Limitations of antifactor Xa
While antifactor Xa testing can provide more consistent and reliable measures of anticoagulation in hospitalized patients anticoagulated with UFH, the test does have some important limitations (see Table 1). Because the assay is a colorimetric assessment, substances that interfere with absorbance of 405 nm light can produce spurious results.2 Common interfering substances include bilirubin (hyperbilirubinemia with Tbili greater than 6.6 mg/dL) including increased bilirubin due to sample hemolysis, and triglyceride (triglyceride level greater than 360 mg/dL). A grossly lipemic sample can be subjected to increased centrifugation to achieve a valid antifactor Xa result; samples with significantly elevated bilirubin should not be tested, and aPTT testing is preferred.3
Importantly, patients receiving direct oral anticoagulants (DOAC) designed to inhibit factor Xa activity (e.g. apixaban, rivaroxaban, edoxaban, betrixaban) may have spuriously high antifactor Xa activity.
A baseline one-time aPTT at the time of heparin therapy initiation is still indicated (in addition to an antifactor Xa assay) as a screening for other coagulation deficiencies.
Finally, drawing a sample from a heparinized line without a proper flush can lead to spurious results for obvious reasons (see Table 2).
Table 2: Differences in interferences of assays for aPTT and Antifactor Xa.
| Key points of Antifactor Xa Assay at UC Davis Medical Center | |
| Critical high value | Greater than or equal to 2.0 U/mL |
| Interference from hemoglobin | Greater than 300 mg/dL |
| Interference from bilirubin | Greater than 20 mg/dL |
| Interference from triglycerides | Greater than 800 mg/dL |
Adapted from Vandiver, et al.
References
- Henry's Clinical Diagnosis and Management. 22nd Edition ed. Philadelphia: Elsevier; 2011.
- Haemostasis: Methods and Protocols. New York: Springer; 2013.
- Jeremy W. Vandiver PD, Vondracek, Thomas G, Pharm D. Antifactor Xa Levels versus Activated Partial Thromboplastin Time for Monitoring Unfractionated Heparin. Pharmacotherapy. 2012;32(6):546-558.
- Labs MM. Anti-Xa Assay for Heparin Monitoring [Hot Topic]. In: Theresa N. Kinard M, ed2017.



