Peter M. Conner, M.D., Pathology Resident and Anna M. Romanelli, Ph.D., Medical Director of Clinical Microbiology Laboratory
INTRODUCTION
Health care providers around the world face the growing challenge of rising rates of resistance of bacterial pathogens to antibiotics. This is particularly pronounced in the management of wound infections, where antibiotics play a key role in treating infections (1). Wound infections have become a significant healthcare burden, contributing to increased morbidity and mortality, prolonged hospitalization, limb loss, and higher medical costs. (6) Wounds can be the source of infection by allowing unconstrained entry of microorganisms into the body, including antimicrobial-resistant bacteria. What’s more, they pose a potential sepsis risk for patients. Most chronic wounds are colonized by polymicrobial aerobic-anaerobic microflora, potentially requiring broad spectrum antibiotics. Chronic wounds can take weeks or months to heal, and some clinicians think they should continue antibiotic therapy until healing occurs; however, there is no evidence to support this belief. As chronic wounds are often reinfected, this can lead to patients being exposed to repeated long courses of therapy. (12)
In addition, the development of new antimicrobials (particularly antibiotics) is not keeping pace with the evolution of resistant microorganisms and novel ways of addressing this problem are urgently required. One such initiative has been the development of antimicrobial stewardship programs (ASPs), which educate healthcare workers, and control the prescribing and targeting of antimicrobials to reduce the likelihood of antimicrobial resistance (13). Antimicrobial stewardship is a coordinated program that promotes the optimal selection, dosage and duration of antimicrobials, resulting in improved patient outcomes, reduced microbial resistance, and decreased spread of infections caused by multidrug-resistant organisms (1). Of great importance has been the European Wound Management Association (EWMA) in supporting ASPs by providing practical recommendations for optimizing antimicrobial therapy for the treatment of wound infections.
In this blog, we will discuss the core elements of antibiotic stewardship programs and how clinicians and the clinical microbiology laboratory can work together to optimize the identification and treatment of clinically relevant wound infections.
ANTIMICROBIAL STEWARDSHIP GOALS
The first goal of a successful antimicrobial stewardship program (ASP) is to implement a multidisciplinary approach in assembling a stewardship team to include an infectious disease physician, a clinical pharmacist with infectious diseases training, infection preventionist, and a close collaboration with the staff in the clinical microbiology laboratory (2). Joseph and Rodvold (14) wrote about the “4D’s of optimal antimicrobial therapy”: right Drug, right Dose, De-escalation to pathogen directed therapy, and right Duration of therapy. The optimal care of an infected patient means treating with the correct, properly dosed antibiotic and one that has the least likelihood of collateral damage (14).
The second goal is to prevent antimicrobial overuse, misuse and abuse. In both the hospital and the outpatient setting, physicians sometimes use antibiotics when they are not necessary. Antibiotics are given to patients with viral infections, noninfectious processes (a classic example is the febrile patient with pancreatitis), bacterial infections that do not require antibiotics (such as small skin abscesses that will resolve with incision and drainage), and bacterial colonization (as in the case of a positive urine culture result in a patient with a bladder catheter). Antibiotics are also frequently misused, such as in the very common scenario of the use of broad-spectrum antibiotics that cover multi-drug resistant organisms in a patient whose infection was acquired in the community or the failure to adjust antibiotics according to culture data, thus maintaining the patient on a regimen to which the organism is not susceptible (3).
The third goal is to minimize the development of resistance. Both at the individual patient level and at the community level, antibiotic use changes susceptibility patterns. Patients exposed to antibiotics are at a higher risk of becoming colonized or infected by resistant organisms (8).
Education, Education, Education! All successful Antimicrobial Stewardship Programs (ASPs) include an educational component. Clinicians are educated about the use of antimicrobials during the process of reading the order sets and treatment algorithms (established by ASP members), during telephone conversations with the antimicrobial steward for the purpose of obtaining authorization for use of a restricted antimicrobial, during interaction with the antimicrobial steward conducting concurrent review and feedback, and through formal didactic sessions or Grand Rounds–type lectures.
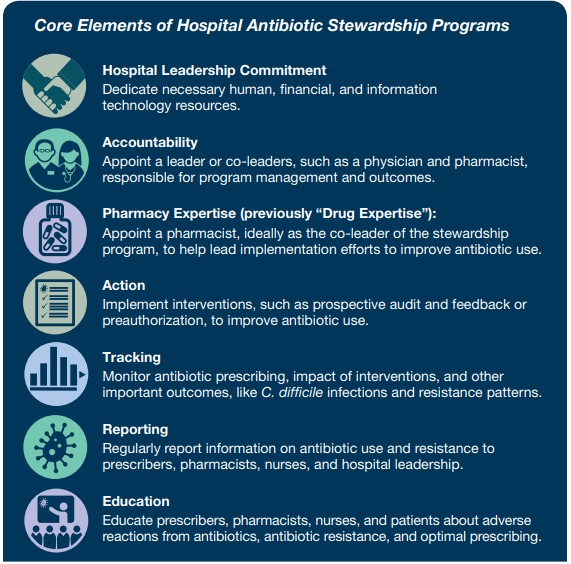
REVIEW OF THE CORE ELEMENTS OF A STEWARDSHIP PROGRAM
The Core Elements are broken down into multiple categories, with mission statements as follows:
The microbiology laboratory plays a critical role in supporting microbiology stewardship in the hospital setting by collaborating with the institutions core ASP team members to help guide the proper use of tests, optimize antibiotic prescribing, and help guide discussions on implementations on the use of rapid diagnostic tests and antibacterial susceptibility tests that might impact antibiotic use (3).
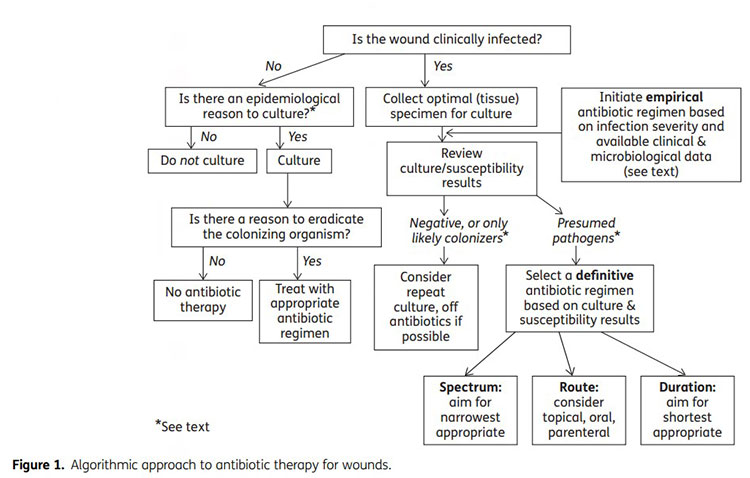
ALGORITHMIC APPROACH TO ANTIBIOTIC THERAPY FOR WOUNDS
The first step in this process is to determine whether a wound is clinically infected. Key diagnostic features include erythema, warmth around the wound, swelling or induration, pain and tenderness, and pus/purulent secretions. In certain patients with neuropathy, vascular insufficiency, or immunodeficiency, they may have abnormal inflammatory responses, potentially masking the classic features and requiring clinicians assess for ‘secondary’ signs of possible wound infections such as: friable or discolored granulation tissue, pocketing, undermining of the ulcer rim, or foul odor. As wound infections are typically local, systemic symptoms are often absent. (12) Once a wound is suspected of being infected, the next step is to properly collect and transport a wound culture.
As cultures usually take at least around 12-24 hours before preliminary results can be reported, empiric antibiotic therapy is sometimes required. Key factors in selecting empirical therapy include assessing the severity of the infections, reviewing the clinical history, and knowing the local antibiotic resistance data (often found in your local institutions AMS files). If a wound is clinically infected, then system antibiotic agents are recommended over topicals. While topical agents are useful for superficial skin infections, there is limited evidence of their effectiveness in wound infections and may even cause discomfort and impair wound healing. Non-antibiotic anti-septic agents may be helpful for localized infections of chronic wounds by suppressing biofilm formation, however, they also may delay healing. Cleaning and debriding of necrotic or inflammatory tissue may accelerate healing and should be performed when appropriate (12).
Systemic antibiotic therapy is the standard treatment for clinical wound infections, but antimicrobial stewardship can be used to support optimal antibiotic use. Several interventions used in antimicrobial stewardship are antibiotic "time outs," the use of newer antibiotics, switching from intravenous to oral agents, and antibiotic de-escalation (4).
CULTURE: A VALUABLE TOOL IN WOUND CARE IF USED CORRECTLY
For a patient with a suspected wound infection, cultures are important in diagnosing the infection, identifying the specific organism, and determining the number of organisms present. This information guides appropriate antibiotic treatment and is crucial in preventing antibiotic resistant infections.
Proper collection of specimens allows for avoidance of contamination with normal flora. Additionally, prompt transport to the lab for processing is essential to the production of meaningful culture results. For wound cultures, the preferred samples are tissue (either by curettage or biopsy) or aspirates. Swabs from wounds are also usually acceptable if properly collected and transported in the correct media; however, issues can arise due to drying, aeration of material, and small quantity collected. To avoid false negatives, samples should be obtained prior to starting antibiotic therapy.
Once at the lab, located in the Specialty Testing Center, the specimen is accessioned and processed. The culture protocol can be broken down into the following categories:
- Direct examination of the specimen
- Plating and Incubation
- Purification and Identification
- Antimicrobial sensitivity testing
Direct examination of the specimen not only involves multiple factors. Pre-processing checkpoints include verifying that the specimen is properly labeled and that an acceptable specimen in the proper media was sent in a timely manner. After a specimen has been determined to be acceptable, a direct smear and gram stain of the clinical material is set up. Direct Gram stains provide for the most rapid method of judging specimen quality and detecting any pathogen(s) in clinical specimens. The Gram reaction, morphology, and arrangement of the organisms, tissue, and inflammatory cells and the specimen type give the physician clues to the preliminary identification and significance of the organisms.
After the initial Gram stain, multiple agar plates are inoculated with samples from the specimen and allowed to be incubated overnight (usually 18-24 hours). The following day, the plates are examined for growth. Various characteristics of the colonies are examined such as their amount, color, shape, and odor. Then one or more colonies are selected for further identification work-up.
At UC Davis Medical Center, most bacterial culture identification is performed using MALDI-TOF mass spectrometry. This relatively new tool allows for rapid, sensitive, and economical identification of bacteria and some fungi. One of the limitations of MALDI-TOF at this time is that it generally requires a pure sample (one microbe) for a successful test. Therefore, if the initial incubation reveals multiple organisms, the top two most abundant organisms are usually purified and identified.
Following identification, determination of the best antimicrobial therapy is often a central aspect for treating the infection. Antimicrobial agents differ in their spectrum of activity and clinical efficacy with respect to the organisms identified, site of infection, and individual causative strain. Antimicrobial susceptibility testing is the traditional in vitro method used to assess bacterial response to antibiotic(s), but testing may also produce results that are inaccurate or misleading. For these reasons, the UCD microbiology lab developed protocols to determine which antimicrobial agents are routinely tested for a given organism or site of infection. These protocols were derived from a combination of national laboratory consensus standards (CLSI), UCDH lab protocols, pharmacy formularies, physicians, and pharmacists. Following these guidelines allows the lab to provide treatment options, while also practicing antimicrobial stewardship so that patients are not given inappropriate medication which can lead to the development of multi-drug resistant organisms.
SUMMARY OF ALGORITHMIC APPROACH
Below is a summary of the algorithmic approach to antibiotic therapy for wounds.
CONCLUSION
The world is facing a worsening crisis related to antimicrobial resistance. As new classes of antibiotics haven’t been developed in recent decades, this has necessitated the creation of antimicrobial stewardship programs (ASPs) to prevent overuse of antibiotics and slow the increase of antibiotic resistant organisms. Antimicrobial stewardship programs and clinical microbiology labs, in partnership with clinicians are crucial for diagnosing and determining the best treatment for chronic wound infections, one of the most common settings in which antibiotics are prescribed. Ultimately, if we all work together to develop and follow the principles of antibiotic stewardship, then we will be able to “maximize benefits, minimize risks to individuals and public health and provide the greatest value for our healthcare dollar.” (7)
Other tools are also available, such as antibiograms, formularies, and guidelines for specific sites and disease states. These can all be found on the UCDMC intranet by searching for the Antibiotic Stewardship program, or by clicking this link.
References:
- Core Elements of Antibiotic Stewardship
- Core Elements of Hospital Antibiotic Stewardship Programs
- The Core Elements of Hospital Antibiotic Stewardship Programs, 2019
- Measuring Drug Susceptibility
- Kowalska-Krochmal B, Dudek-Wicher R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens. 2021;10(2):165. Published 2021 Feb 4.
- Nussbaum, Samuel R., et al. “An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds.” Value in Health, vol. 21, no. 1, 2018, pp. 27–32.
- Antibiotic Stewardship Program
- Edward Stenehjem, Adam L Hersh, Whitney R Buckel, Peter Jones, Xiaoming Sheng, R Scott Evans, John P Burke, Bert K Lopansri, Rajendu Srivastava, Tom Greene, Andrew T Pavia, Impact of Implementing Antibiotic Stewardship Programs in 15 Small Hospitals: A Cluster-Randomized Intervention, Clinical Infectious Diseases, Volume 67, Issue 4, 15 August 2018, Pages 525–532.
- Diego Viasus, Milly Vecino-Moreno, Juan M. De La Hoz & Jordi Carratalà (2017) Antibiotic stewardship in community-acquired pneumonia, Expert Review of Anti-infective Therapy, 15:4, 351-359.
- Philip Zachariah, Lisa Saiman, Expanding antimicrobial stewardship strategies for the NICU: Management of surgical site infections, perioperative prophylaxis, and culture negative sepsis, Seminars in Perinatology, Volume 44, Issue 8, 2020, 151327, ISSN 0146-0005.
- Michael Miller, Poorly Collected Specimens May Have a Negative Impact on Your Antibiotic Stewardship Program, Clinical Microbiology Newsletter, Volume 38, Issue 6, 2016, Pages 43-48, ISSN 0196-4399.
- Lipsky, Benjamin A., et al. “Antimicrobial Stewardship in Wound Care: A Position Paper from the British Society for Antimicrobial Chemotherapy and European Wound Management Association.” Journal of Antimicrobial Chemotherapy, vol. 71, no. 11, 2016, pp. 3026–3035.
- Edwards-Jones, Val. “Antimicrobial Stewardship in Wound Care.” British Journal of Nursing, vol. 29, no. 15, 2020.
- Joseph J, Rodvold KA. The role of carbapenems in the treatment of severe nosocomial respiratory tract infections. Expert Opin Pharmacother. 2008;9(4):561-575