Maryna Vazmitsel, MD, Elham Kamangar, MD, and Alaa Afify, MD
Introduction
The touch preparation (TP) technique is a tissue preparation method in which a fresh tissue will exfoliate cells on a glass slide after touching that slide. It is usually performed on core needle biopsies (CNB) during rapid onsite evaluation (ROSE) to confirm their adequacy and appropriately triage samples. The purpose of this study was to assess our experience with TP of lung CNB and to determine if any improvement is necessary.
Material and Methods
Lung CNB were identified via the University of California Davis Medical Center’s laboratory information system in a six-month period during 2021. CNBs were obtained either under CT guidance or US guidance in the radiology department or bronchoscopy unit of the pulmonology department. Cytotechnologists were on-site and immediately prepared a touch preparation imprint of the obtained core biopsy. The TP sample was alcohol fixed, toluidine blue-stained, and examined microscopically to determine the adequacy of the biopsy. The tissue biopsy specimens were routinely processed, and 4-micron sections of the tissue were stained with hematoxylin and eosin and reviewed under the microscope by a pathologist who rendered the final diagnosis.
Cases in which TP of CNB was performed were retrieved and reevaluated by the pathologist. The results were tabulated and compared. Cases with discrepancies were determined.
Results
A total of 109 CNBs obtained from lung lesions were performed, including 86 CT-guided (78.9%) and 23 US-guided. In 23 cases, TP of CNB was performed and reviewed to ensure specimen adequacy. Cytotechnologists designated 13 TP cases as adequate and 10 cases as inadequate. The final diagnoses of tissue biopsies for corresponding adequate and inadequate TP cases are summarized in Table 1. We agree with the interpretation of core needle biopsy in all cases.
Table 1. Correlation of TP adequacy result with biopsy diagnosis (n=23)
Diagnosis based on core needle biopsy |
Cytotechnologist interpretation of TP at ROSE |
|
Adequate for evaluation |
Inadequate for evaluation |
|
| 1. Benign/reactive change | 1 | 6 |
| 2. Benign neoplasm | 1 | |
| 3. Atypical findings | 2 | 3 |
| 4. Suspicious for malignancy | 1 | |
| 5. Malignant neoplasm | 8 | 1 |
| Total | 13 | 10 |
The final diagnoses of lung CNB for the cases in which TP were performed are summarized the Histogram 1 and Histogram 2.
When cytotechnologists issued TP as adequate for interpretation based on the review at ROSE, it was 100% accurate. In contrast, when the cytotechnologist designated the TP as inadequate for evaluation, it revealed benign/reactive changes in 60%, atypical/metaplastic changes in 30%, and malignant neoplasm in 10%.
A review of TP for this false-negative case revealed very few atypical cells and therefore considered a false negative case. The corresponding lung CNB of this case revealed angiosarcoma, which is a very rare malignant tumor and difficult to diagnose even by an experienced pathologist. Additionally, the tumor was very small (2.0 mm), and most of the CNB consisted of benign lung tissue. In three cases categorized by cytotechnologists on TP as inadequate, the corresponding lung CNB showed atypical pneumocytes and/or metaplastic changes in a minute area of CNB. In these cases, a review of TP revealed very few pneumocytes and air-drying artifacts.
In 6 lung CNB diagnosed as benign and interpreted as inadequate by cytotechnologists, a review of the TP showed very low cellularity of the TP sample, core biopsy showed benign lung parenchyma and fibrosis.
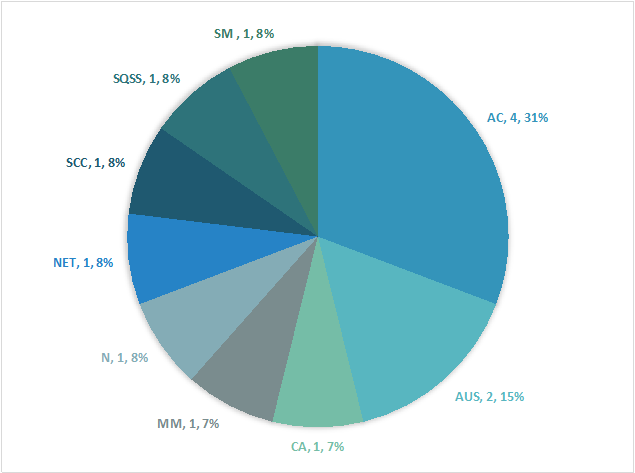
AC – adenocarcinoma; ASA – angiosarcoma; CA - non-small cell carcinoma, NOS; SCC - small cell carcinoma; SQCC - squamous cell carcinoma; MM - malignant melanoma; SM – suspicious for malignancy; NET – neuroendocrine tumor; AUS – atypical pneumocytes/metaplastic change; N – negative for malignancy, benign lung parenchyma.
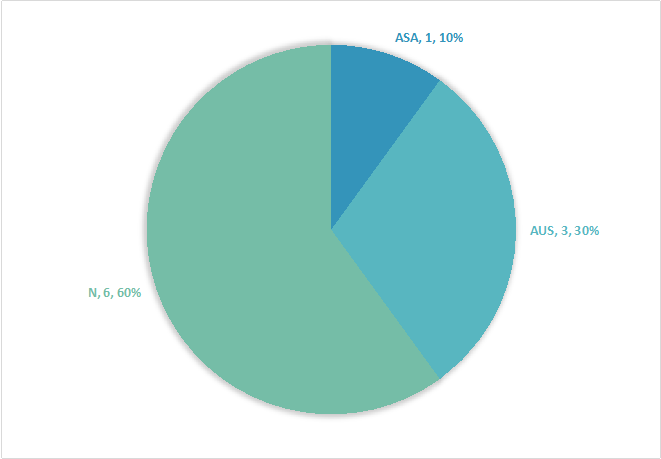
ASA – angiosarcoma; AUS – atypical pneumocytes/metaplastic change; N – negative for malignancy, benign lung parenchyma.
Discussion
The touch preparation technique was first introduced into the medical literature by Dudgeon and Patrick in 1927 [1]. TP is performed on small biopsies, such as core needle biopsies, during ROSE to confirm their adequacy and appropriately triage samples. In addition, the ROSE of TPs helps reduce the number of cores needed to obtain diagnostic material [2; 3]. The great advantage of TP, especially in lung biopsies, is to avoid pneumothorax or injury of large vessels.
Our study clearly showed that when a cytotechnologist designates the TP specimen as adequate, the corresponding tissue biopsy shows diagnostic material in 100% of cases. Based on our study, lung core biopsy corresponding to inadequate TP revealed benign lung parenchyma (60% cases) or atypical pneumocytes/metaplastic changes (30% cases). We found that the small size of the CNB, the small size of diagnostic tissue, or the fibrotic nature of the tissue contributed to the inadequate TP samples. Benign processes do not exfoliate abundant cells and yield few cells on the touch preparation sample.
The skills of the person preparing the TP play a significant role in the quality and adequacy of the TP [4; 5]. Our study revealed that our cytotechnologists, who prepared the sample, were accurate in 100% of cases designated to adequate.
This study is based on a small number of cases, and further study will be done.
Conclusion
Adequate touch preparation is reliable in determining the malignant diagnosis. In contrast, the inadequate sample is most probably contributed to the benign, reactive lesions or small size of the represented tissue.
References
- A new method for the rapid microscopical diagnosis of tumours, with an account of 200 cases so examined. Dudgeon LS, Patrick CV. 1927, Br J Surg., pp. 15:250–261.
- Pantanowitz L, Xing J. Intraoperative cytology. In: Pantanowitz L, Xing J, Monaco SE, eds. Atlas of Touch Preparation Cytopathology. New York: NY: Demos Medical, 2017.
- Impact of touch imprint cytology on imaging guided core needle biopsies: an experience from a large academic medical center laboratory. Li Z, Tonkovich D, Shen R. 2016, Diagn Cytopathol., pp. 44:87–90.
- Imprint cytopathology of core needle biopsies: a “first-responder” role of cytotechnologists. Thomas SV, Lagana A, Dittmer KM, Wakely PE. 2015, J Am Soc Cytopathol., Vol. 4, pp. 16–24.
- American Society of Cytopathology Clinical Practice Committee. Results from the 2019 American Society of Cytopathology survey on rapid on-site evaluation—Part 1: objective practice patterns. VanderLaan PA, Chen Y, Alex D, et al. 2019, J Am Soc Cytopathol., Vol. 8, pp. 333–341.



