Aqiba Bokhari MD, Zarir E. Karanjawala MD, Denis M. Dwyre MD
Flow cytometry (FCM) by immunophenotypic analysis has a prominent role in the diagnosis and classification of hematolymphoid neoplasms, and in monitoring response to therapy. There have been tremendous advances for almost two decades in FCM instrumentation and its multiparametric nature that has allowed identification of different normal cell populations, and in identifying immunophenotypic aberrancies even when only few cells are analyzed. FCM characterizes both surface and cytoplasmic protein expression. The information obtained from FCM data establishes the diagnosis of mature lymphoid neoplasms, immature blastic malignancies, maturing myeloid and monocytic malignancies, and plasma cell neoplasms. As FCM is complementary in the evaluation of hematolymphoid neoplasms, additional ancillary testing, including immunohistochemistry, conventional cytogenetic, fluorescence in situ hybridization (FISH), and molecular diagnostic studies can also be undertaken based on the flow data. FCM is highly sensitive in the detection of minimal residual disease (on the order of 1 in 104 to 106). It also identifies therapeutic targets on the surface of malignant cells for potential antibody-based directed therapies such as rituximab (anti-CD20), epratuzumab (anti-CD22), gemtuzumab (anti-CD33), and blinatumomab (directed against CD19 and CD3) [1,2].
In a flow cytometer, the individual cells in suspension rapidly pass through a series of finely focused lasers. As the cell passes through the laser beam, it simultaneously scatters the light at a low angle (also called forward scatter “FSC”) proportional to the cell volume/size, as well as at a high angle (also called side scatter “SSC”) proportional to the cell’s complexity (type and amount of cytoplasmic granularity, cytoplasmic membrane irregularities, and nuclear characteristics). Cells are further characterized by concurrent staining with multiple antibodies conjugated to various fluorochromes (colors). If a cell expresses a specific antigen bound to a fluorochrome-conjugated antibody, the fluorochrome emits light at a particular wavelength measured by detectors. Many clinical laboratories use 6- to 8-color FCM, with some using 10 or more colors. The minimal acceptable amount is 4-color FCM analysis to ensure reliable discrimination of neoplastic cell populations in various sample types. However, it is important to have a detailed knowledge of normal antigen expression of all the lineages and their light scatter characteristics to be able to differentiate malignant cell populations [2,3].
Clinical FCM analysis can be performed on blood, bone marrow, lymph node, extranodal tissue biopsies, fine-needle aspirates (FNA), and body fluids (CSF, pleural, peritoneal). The following are essential: 1) Timely processing of samples. 2) Collection of blood and bone marrow specimens in an appropriate anticoagulant. 3) Removal of excess erythrocytes preferably by lysis. 3) Submission of several core biopsies in cases of a bone marrow “dry tap”. 4) Mechanical disaggregation of tissue into cell suspensions with commercial devices or manual tools. 5) Rapid CSF preservation.
Viability of cells is important as nonviable cells may nonspecifically bind antibodies and interfere with accurate immunophenotyping. General guidelines suggest to reject non-irreplaceable samples with less than 75% viability. A sample should not be regarded as a true negative if a neoplastic process could not be identified because of poor viability as subsequent testing may render useful results with a viable specimen.
Diagnosis of lymphoma by FCM is frequently made on small samples including small biopsies, FNA and body fluids (CSF, vitreous humor, effusions). FCM has increased sensitivity for detection of lymphoid neoplasms such as chronic lymphocytic leukemia, mantle cell lymphoma, lymphoplasmacytic lymphoma, Burkitt lymphoma, and plasmacytoma. FCM is significantly more sensitive than cytology alone in detection and prognostication of non-Hodgkin’s lymphoma and CNS involvement by leukemia [2].
Consensus Recommendations on FCM (Bethesda, Maryland 2006) (1,4)
The World Health Organization (WHO) classification for tumors of the hematopoietic and lymphoid tissues endorses a multiparametric approach to diagnosis and outlines the morphologic, immunophenotypic, and genotypic features characteristic of each disease entity. However, it is neither essential nor cost-effective to perform multiple studies on every specimen. Therefore, in 2006, a group of international experts met in Bethesda, Maryland, to formulate consensus recommendations for flow cytometric immunophenotypic analysis of hematolymphoid neoplasms. In contrast to the previous consensus meetings that had considered the flow cytometric evaluation of specimens based on the “reagents” required to evaluate a specific disease entity, the Bethesda group took a more practical approach and reached a consensus that flow cytometric immunophenotyping should be indicated based on the “clinical presentation” as follows:
- In clinical situations, where FCM serves as a rapid sensitive screening tool for the presence and further classification or absence of hematologic malignancy:
- Cytopenias, especially bicytopenia and pancytopenia
- Elevated leukocyte count, including lymphocytosis, monocytosis, and eosinophilia
- The presence of atypical cells or blasts in the peripheral blood, bone marrow, or body fluids
- Plasmacytosis or monoclonal gammopathy
- Organomegaly and tissue masses
- FCM acts as a useful tool for:
- Staging a previously diagnosed hematolymphoid neoplasm
- Monitoring response to treatment including detection of minimal residual disease (MRD)
- Documenting relapse or progression
- Diagnosing a hematologic malignancy, such as a therapy-related myelodysplastic syndrome (MDS)
The Bethesda group also agreed that FCM is not indicated in the following situations:
- Mature neutrophilia
- Polyclonal hypergammaglobulinemia
- Polycythemia
- Thrombocytosis
- Basophilia
The international consensus was also reached on the cell lineages to be evaluated and the number of antigens/reagents to include in a primary evaluation of each lineage, which may be guided by clinical presentation (1,5). Reagents of clinical utility in the evaluation of various hematolymphoid neoplasms include:
Mature and Immature B-cell lymphoid neoplasms: CD5, CD10, CD19, CD20, CD45, surface Kappa and Lambda, CD11c, CD15, CD22, CD23, CD25, CD13, CD33, CD34, CD38, CD43, CD79a, CD79b, CD103, FMC-7, cytoplasmic Kappa and Lambda, ZAP-70, TdT
Mature T- and NK-cell lymphoid neoplasms: CD2, CD3, surface, CD4, CD5, CD7, CD8, CD45, CD56, CD1a, cCD3, CD10, CD16, CD25, CD26, CD30, CD57
Plasma cell disorders: CD19, CD38, CD45, CD56, CD10, CD117, CD138, cytoplasmic Kappa and Lambda
Myeloid and monocytic neoplasms: CD11b, CD13, CD14, CD15, CD16, CD33, CD34, CD45, CD56, CD117, HLA-DR, CD2, CD4, CD7, CD25, CD36, CD38, CD41, CD61, cCD61, CD64, CD71, cMPO, CD123, CD163, CD235a
Examples of Case Studies:
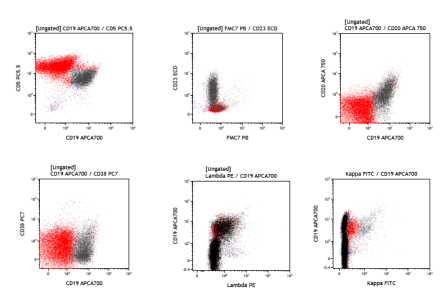
1. Chronic lymphocytic leukemia (CLL/SLL): The patient is a 67-year-old female with history of CLL, who presents with right cervical lymphadenopathy. A right level 5 cervical lymph node tissue flow cytometry (FCM) study is performed, which shows a population of monotypic B-cells with CD5+/CD23+ co-expression, CD19+, subset dim CD20+, dim surface lambda+, and CD38-, FMC7-, surface kappa (equivocal), subset CD49d-, consistent with CLL/SLL.
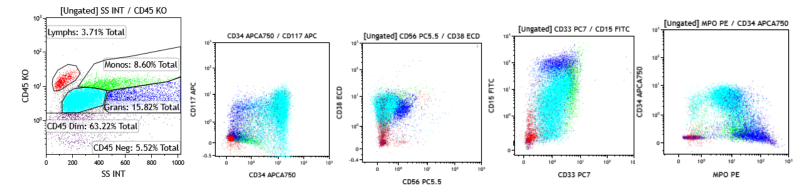
2. Acute myeloid leukemia (AML): The patient is a 14-year-old female who presents with malaise and bleeding gums. Her CBC showed leukocytosis, normocytic anemia, and thrombocytopenia. Review of the peripheral blood smear shows blasts. A bone marrow FCM study is performed, which shows approximately 60% abnormal myeloblasts CD34+, CD117+, CD38+, CD15+, and MPO+, and CD56-, CD10-, CD20-, and CD138-, consistent with AML.
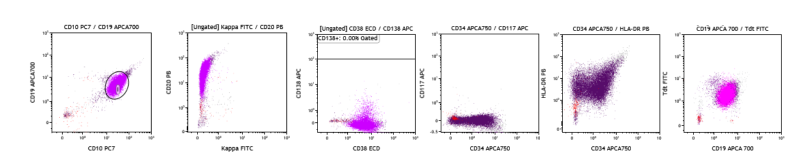
3. B-Acute Lymphoblastic Leukemia (B-ALL): The patient is a 31-year-old female who presents for evaluation of pancytopenia. A bone marrow FCM study is performed, which shows approximately 90% abnormal B-lymphoblasts CD19+, CD10+, CD20+, CD38+, CD34+, TdT+, HLA-DR+, and Kappa-, lambda-, CD117-, CD56-, CD3-, MPO-, CD45-, consistent with B-ALL.
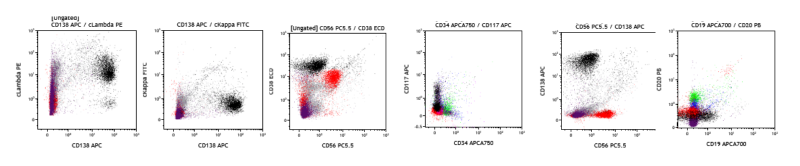
4. Plasma Cell Neoplasm (PCN): The patient is an 86-year-old female who presents with anemia, bone pain, lytic bone lesions, renal failure, and hypercalcemia. A bone marrow FCM study is performed, which shows approximately 12% of cytoplasmic lambda restricted (cLambda+) plasma cells which are CD138+, CD38+, subset CD117+, and CD19-, CD20-, CD56-, cKappa-, consistent with Lambda-restricted PCN.
References
- Fiona E Craig and Kenneth A Foon. Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008;111(8):3941-67.
- Elaine S. Jaffe et al. Hematopathology; second edition (2017). Flow cytometry. Chapter 5. 53-67.
- RC Braylan, et al. Optimal number of reagents required to evaluate hematolymphoid neoplasias: results of an international consensus meeting. Cytometry. 2001;46(1):23-27.
- BH Davis, et al. 2006 Bethesda International Consensus recommendations on the flow cytometric immunophenotypic analysis of hematolymphoid neoplasia: medical indications. Cytometry B Clin Cytom. 2007;72 (suppl 1):S5-13.
- BL Wood et al: 2006 Bethesda International Consensus recommendations on the immunophenotypic analysis of hematolymphoid neoplasia by flow cytometry: optimal reagents and reporting for the flow cytometric diagnosis of hematopoietic neoplasia. Cytometry B Clin Cytom. 2007;72B:S14-22.



