Ananya Datta Mitra, M.D.
Hooman H. Rashidi, M.D.
Karen Matsukuma, M.D., Ph.D.
Background
Because the gastrointestinal (GI) tract is a site of continuous challenge by foreign antigens, it contains a well-developed immune system component. The upper aerodigestive tract and small and large intestines have endogenous lymphoid tissue, including the Waldeyer ring in the oropharynx, Peyer patches in the terminal ileum, and mucosal lymphoid aggregates in the appendix. In contrast, the esophagus and stomach are not associated with a significant amount of organized lymphoid tissue under normal conditions but can acquire lymphoid aggregates under constant antigenic stimulation, such as in the setting of gastric Helicobacter pylori infection.
The most common specimens encountered by GI pathologists in daily practice are endoscopic biopsies of the upper and lower GI tract. A subset of these will contain lymphoid tissue, which may be mentioned in the pathology report. To aid in understanding what is meant when lymphoid tissue is described in the pathology report, we provide the following definitions:
Lymphoid tissue: A general term to describe a collection of B-cells, T-cells, and support cells. Lymphoid tissue is normally concentrated along the mucosal surfaces of the body (tonsils, Peyer patches) and can also be acquired at sites of chronic antigenic stimulation. The primary lymphoid tissues are bone marrow and thymus (sites of lymphocyte development); the normal secondary lymphoid tissues include mucosa-associated lymphoid tissue (MALT, mentioned above) and the lymph node, which both serve similar functions. In contrast to mucosa-associated lymphoid tissue which is non-encapsulated, a lymph node is a specialized type of lymphoid tissue that is in continuity with the lymphatic system and enclosed within a fibrous capsule.
Other terms used to describe specific types of lymphoid tissue are:
- Lymphoid aggregate/infiltrate: A collection of B cells, T cells, and supporting cells, present within the stroma of various organs. The term can be used to describe endogenous lymphoid tissue or acquired lymphoid tissue.
- Lymphoid follicle: Similar to a lymphoid aggregate (sometimes used interchangeably) but typically refers to a more discrete collection of B cells, T cells, and supporting cells. There are two types of lymphoid follicle:
- Primary follicles are lymphoid follicles that do not yet contain a germinal center (described below). They are precursors to secondary follicles and are composed predominantly of small naïve B cells and inconspicuous supporting cells. Because primary follicles appear monotonous, they can occasionally raise concern for lymphoma.
- Secondary follicles contain germinal centers. The presence of a germinal center indicates activation of adaptive (antigen-specific) immunity and typically is a feature of a reactive rather than a neoplastic process.
- Germinal center: The site where antigen-presenting cells interact with naïve B-cells to initiate an antigen-specific immune response. It is the slightly paler circular area within the secondary lymphoid follicle (Figure 1) and is composed predominantly of B cells, with rare T-cells and scattered support cells. Of note, the darker area surrounding the germinal center (the mantle zone) consists predominantly of B-cells and can be thought of as residual primary follicle surrounding the germinal center.
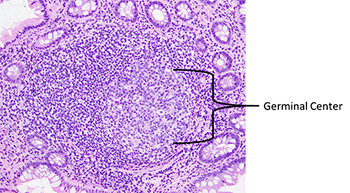
Terms frequently used in GI pathology reports
Reactive lymphoid aggregate: A lymphoid aggregate that demonstrates a germinal center. The presence of a germinal center is typically indicative of a reactive rather than a neoplastic process (but should be taken in the context of the entire case).
Prominent lymphoid aggregate: The term “prominent” is a descriptor often used to describe a lymphoid aggregate in GI mucosa that is larger than expected (thus possibly accounting for the endoscopic impression of a polyp). Unless otherwise stated, when composed of primary or secondary follicles, there are no features worrisome for lymphoma.
Atypical lymphoid aggregate: A lymphoid aggregate that lacks the typical morphologic and immunophenotypic features of a reactive lymphoid aggregate but is not diagnostic of lymphoma.
How does a pathologist distinguish a reactive lymphoid aggregate from lymphoma?
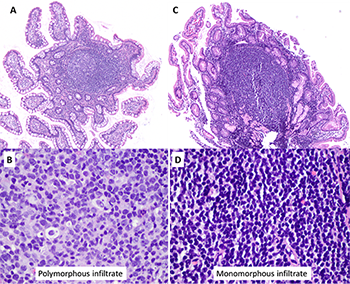
Some histologic features can help to distinguish reactive lymphoid aggregates from lymphoma (Table 1, Figure 2). However, it is essential to keep in mind that distinction between a reactive and a neoplastic lymphoid process is often not possible based on morphology alone. Moreover, certain lymphomas (e.g., extranodal MALT lymphoma) may develop in association with reactive lymphoid infiltrates, especially in the stomach. Thus, immunohistochemistry, gene rearrangement studies, and other ancillary studies are frequently necessary for further evaluation.
Table 1. Histologic features that help distinguish reactive lymphoid aggregates from lymphoma.
Histologic featureReactive lymphoid aggregateLymphomaSizeUsually smallUsually LargerArchitecture of the GI mucosaPreserved mucosal architecture with lymphoid follicles sitting above the muscularis mucosaeDisrupted mucosal architecture with lymphoid cells pushing aside or destroying glands (or crypts) and/or disrupting the muscularis mucosaeGerminal centersPresentTypically, absentCytologyPolymorphous: Mixture of large and small cells, including plasma cells; minimal cytologic atypiaMonomorphous: Cells typically are similar in size and shape; may show nuclear membrane irregularitiesLymphoepithelial lesions(lymphocytes invading and destroying glands or crypts)AbsentCan be present
What does a pathologist do when encountering a lymphoid aggregate in the GI mucosa?
The following is the algorithm commonly followed here at UC Davis for evaluation of a lymphoid aggregate found in the GI tract (Figure 3):
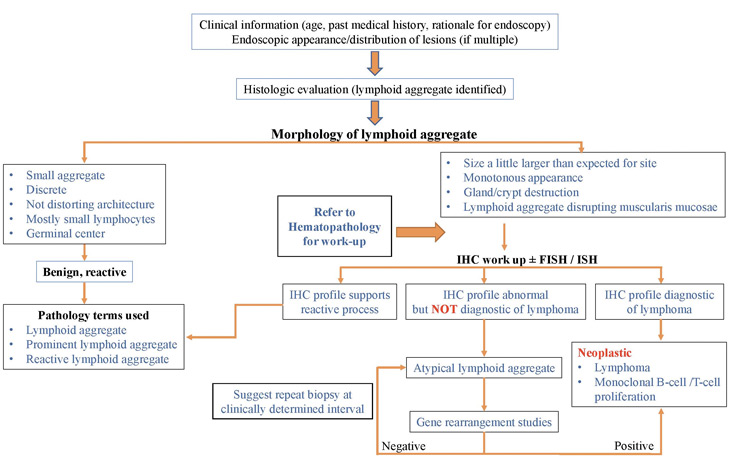
IHC: Immunohistochemistry
FISH: Fluorescence in situ hybridization
ISH: In situ hybridization (RNA)
Monoclonal B-cell/T-cell proliferation: An abnormal collection of B or T-cells demonstrating clonality but not always diagnostic of a specific lymphoma.



