UC Davis Health High Sensitivity Cardiac Troponin Implementation Workgroup
Introduction
Cardiac troponin (cTn) is the preferred biomarker for diagnosing acute myocardial infarction (MI).1 As defined by the 3rd Universal Definitions, the diagnosis of MI is based on a rise and/or fall in a cardiac biomarker (preferably cTn) with at least one value above the 99th percentile of the upper reference limit (URL) and at least one of the following: (a) symptoms of ischemia, (b) new or presumed new significant ST-segment-T wave (ST-T) changes or new left bundle branch block, (c) development of pathological Q waves in the electrocardiogram, (d) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality, or (e) identification of an intracoronary thrombus by angiography or autopsy.
Another recommendation of the 3rd Universal Definition includes changes in cTn values be defined based on the assay's imprecision.1 Imprecision is the reproducibility of a test and is measured as percent coefficient of variation (CV).2 In contrast, accuracy, is how close a result is to the “true value”. For the purpose of this article, we will focus on precision. How imprecision impacts serial testing is as follows. For example, if one measures the same patient sample five times using a perfectly precise test, the same result will occur all five times. Alternately, if the same sample was tested five times using a very imprecise test, results will vary around an average value. The CV describes his variability, and with an imprecise test, it becomes very difficult to discern if cTn changes were biologically true or an artifact of assay imprecision (Figure 1). In other words, a highly precise test is desirable for serial testing since changes in biomarker levels are not attributed to the “analytical noise” of the test. To this end, the 3rd Universal Definition suggests the optimal cTn assay cut off should have a CV of £10% at the 99th percentile URL.1 Assays with a CV > 20% at the 99th percentile URL should not be used.
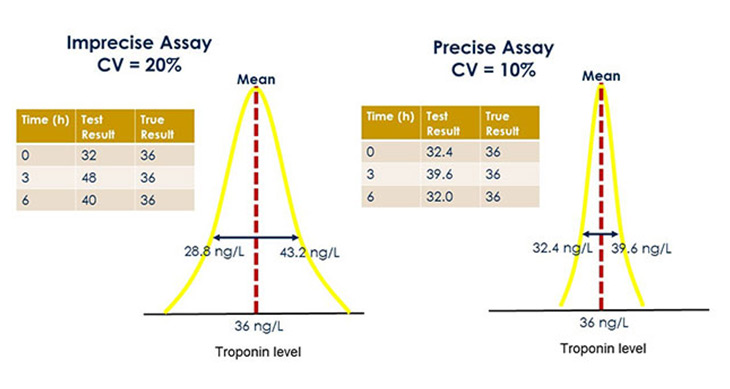
Figure 1. Analytical Imprecision for Troponin Testing. The figure illustrates the relationship of imprecision during serial testing at time 0, 3, and 6 hours. Given, two different troponin assays with analytical imprecision 20% (left) and 10% (right) coefficient of variation (CV), were used for the same patient sample. Also given, the true patient troponin level is 36 ng/L. Assume the 99th percentile of the upper limit of normal to be 40 ng/L. Percent CV describes the percent variability around a given mean. For the imprecise assay, we would expect at least 67% of results to be +/- 20% of the mean and potentially showing a false change in troponin including one value above the 99th percentile. In contrast, the more precise assay (CV = 10%) would have 67% of results vary within +/- 10% around the mean during serial testing—exhibiting minimal variation and without having a value above the 99th percentile.
High Sensitivity Cardiac Troponin Assays
In June 2017, the first Food and Drug Administration (FDA) approved high sensitivity (hs) cTn assay became commercially available in the United States.3 As defined by the International Federation for Clinical Chemistry (IFCC), hs-cTn assays must meet two analytical performance criteria: (1) imprecision of £10% at the 99th percentile of the URL, and (2) ability to quantify cTn levels in ³50% of apparently healthy individuals (Figure 2).4 The benefits of hs-cTn assays include higher diagnostic accuracy for acute MI than contemporary assays,5 1- or 2-hour accelerated diagnostic protocols (ADP) for acute MI.6,7 Unique to the United States, the newly approved hs-cTn assay may also be used with sex-specific 99th percentile cut offs—adding another level of complexity when implementing this new test.3
Clinical Impact of High Sensitivity Cardiac Troponin Testing
In the recent study by Twerenbold et al., a hs-cTnT assay was evaluated in emergency department patients presenting with symptoms suggestive of AMI and extends finding from other similar trials.5,7,8 In particular, Twerenbold et al.5 compared pre- (n = 1,455) and post-hs-cTnT implementation (n = 1,089) cohorts. MI diagnosis occurred more frequently at discharge after hs-cTnT introduction (10 vs. 14%, P < 0.001). Interestingly, coronary angiography rates (23 vs. 23%, P = 0.092) and percentage of coronary angiographies showing no stenosis were similar before and after hs-cTnT implementation. The use of stress testing was also found to be substantially reduced from 29 to 19% (P < 0.001). In outpatients, median time to discharge from the ED decreased by 79 min (P < 0.001). Mean total costs decreased by 20% in outpatients after the introduction of hs-cTnT (P = 0.002). However, it must be noted that sex specific cut offs were not used in this study.
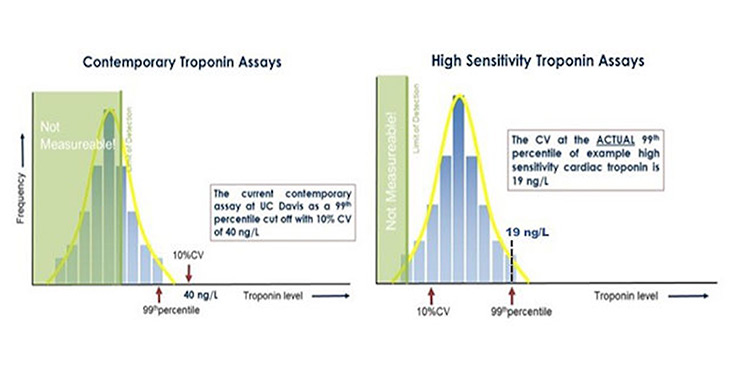
Figure 2. Differences in 99th Percentile Cut-Offs for Contemporary vs. High Sensitivity Assays. This figure represents example assays for educational purposes. The left panel illustrates a contemporary troponin assay, while the left panel illustrates a high sensitivity assay. Contemporary assays are not able to detect troponin >50% of the healthy population. Furthermore, the assay cut off (40 ng/L) where analytical imprecision is <10% is above the actual 99th percentile. In contrast, the high sensitivity assay quantifies troponin in >50% of the healthy population and exhibits an assay imprecision of <10% at the 99th percentile – allowing the actual 99th percentile to serve as the cut off (19 ng/L).
High Sensitivity Cardiac Troponin in the United States
Peer reviewed literature from European hs-cTn studies serve as a strong foundation for assay implementation in the United States. However, several caveats remain. First, ordering practices in the United States are significantly different.9 The frequency of cTn ordering remains higher in the United States—potentially increasing the false positive results when using hs-cTn assays. European experience with sex specific cTn cut offs is also limited and not widely adopted. Interestingly, these sex specific hs-cTnT cut offs also differ between the two regions. For the United States, these cut offs are 14 and 22 ng/L for men and women respectively, with a combined cut off of 19 ng/L.3 In contrast, European sex specific cut offs (9 ng/L for women, and 15.5 ng/L for men) for hs-cTnT have been used in research studies, however, adoption remains limited and most facilities rely on the 14 ng/L combined cut off.10
References
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60(16):1581-98.
- Chesher D. Evaluating assay precision. Clin Biochem Rev. 2008 Aug;29 Suppl 1:S23-6.
- FDA website: https://www.accessdata.fda.gov/cdrh_docs/reviews/K162895.pdf, Accessed on April 5, 2018
- Apple FS, Collinson PO, Biomarkers ITFoCAoC. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58(1):54-61.
- Twerenbold R, Jaeger C, Rubini Gimenez M, et al. Impact of high-sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. Eur Heart J. 2016;37(44):3324-3332.
- Twerenbold R, Boeddinghaus J, Nestelberger T, et al. Clinical Use of High-Sensitivity Cardiac Troponin in Patients With Suspected Myocardial Infarction. J Am Coll Cardiol. 2017;70(8):996-1012.
- Reichlin T, Schinder C, Drexler B, Twerenbold R, Reiter M, Zellweger C, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012;172:1211– 8.
- Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011;135:1367–73.
- American College of Cardiology website article: http://www.acc.org/latest-in-cardiology/articles/2017/08/07/07/46/a-brief-review-of-troponin-testing-for-clinicians, Accessed on April 5, 2018
- Gimenez MR, Twerenbold R, Boeddinghaus J, et al. Clinical effect of sex-specific cutoff values of high sensitivity cardiac troponin T in suspected myocardial infarction. JAMA Cardiol 2016;1(8):912-920



