John Rodrigo, M.D., Postdoctoral Scholar
Fabian Lara, M.D., Postdoctoral Scholar
Saba Ali, M.D., Resident Pathologist
Denis Dwyre, M.D., Director of Hematopathology
Grace Monis, M.D., Special Coagulation
Sarah Barnhard, M.D., Associate Director of Transfusion
Scott Bainbridge, C.L.S., Special Chemistry/Toxicology Supervisor
Nam Tran, Ph.D., Director of Clinical Chemistry and SARC
Background: Antiphospholipid syndrome (APS) is an autoimmune disorder caused by antibodies against membrane anionic phospholipids or their associated plasma proteins.1 Specifically, autoantibodies are formed against epitopes exposed by the physiological binding of certain cellular membrane components, mainly lupus anticoagulant (LA) and cardiolipin (CL), and associated bound proteins, namely apolipoprotein H, predominantly β2-glycoprotein-1 (B2GPI) and prothrombin. Others include protein C, protein S, annexin V.2,3 This condition results in a hypercoagulable state that promotes arterial and venous thrombosis, as well as pregnancy-related complications such as miscarriage, stillbirth, preterm delivery, and severe preeclampsia.
The most common clinical manifestations include deep venous thrombosis of the lower limbs, arterial cerebrovascular thrombosis, and spontaneous abortions.1-4 The current diagnostic criteria for APS require at least one clinical event (i.e., thrombosis or pregnancy complication, and two or more laboratory criteria including the presence of Lupus anticoagulant (LA), anti-cardiolipin antibodies (aCL), and/or anti-B2GPI. Table 1 summarizes the revised classification criteria for APS.5,6
| Table 1. Classification for antiphospholipid syndrome (APS): APS is present if at least one of the clinical criteria and one of the laboratory criteria that follow are met. |
|---|
| Clinical criteria: At least one of the following |
|
| Laboratory criteria: All laboratory criteria should be present on ≥2 occasions, at least 12 weeks apart |
|
Laboratory Best Practice: Previous APS tests were based on non-standardized and laborious enzyme linked immunosorbent assays (ELISA) often performed as laboratory developed tests (LDT). ELISA assay cut-offs for aCL and anti-B2GPI (usually only IgG and IgM) were also not standardized, often relying on poorly defined data from limited studies. As noted in Table 1, the current APS criteria consider aCL and anti-B2GPI values greater than 99th percentile of the normal population as positive.
Today, modern immunoassay techniques have become highly automated and exhibit improved analytical performance. The use of bead-based immunoassay techniques enables rapid simultaneous (“multiplex”) detection of targets such as autoimmune antibodies. These techniques have enhanced anti-nuclear antibody (ANA) testing, and has now been expanded to include aCL and anti-B2GPI. To this end, the UC Davis Medical Center Special Chemistry Section has recently employed the use of Bioplex 2200 (Bio-Rad, Hercules, CA) multiplex APS assay. The Bioplex 2200 is a multi-flow immunoassay platform that can detect human IgM and IgG antibodies, as well as IgA, for both CL and B2GPI. In short, the new Bioplex APS assay retains the clinical sensitivity (i.e., reduced false negatives) of prior methods (96-100%), while improving clinical specificity (i.e., reduced false positives) from 91% (ELISA method) to 95%.7 Avoiding false positives is critical, since the diagnosis of APS carries substantial risk. It must be noted that the diagnosis of APS remains very challenging despite advancements in laboratory testing methods. The recommended testing algorithm for APS is summarized in Figure 1.
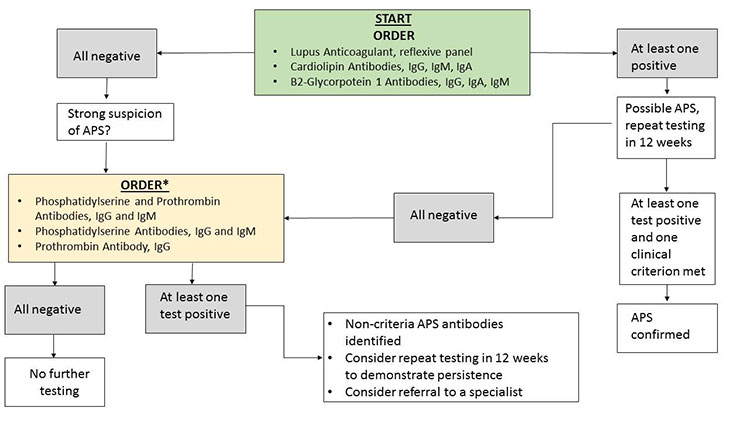
Acknowledgements: We thank the UC Davis Medical Center Department of Pathology and Laboratory Medicine Clinical and Quality Research team for collecting and analyzing APS data.
References
- Pier Luigi Meroni M. Orietta B, Elena R, et al. "Pathogenesis of antiphospholipid syndrome: understanding the antibodies". Nat Rev Rheumatology 2011;7:330
- Bobba RS, Johnson SR, Davis AM. "A review of the Sapporo and revised Sappaoro criteria for the classification of antiphospholipid syndrome". Where do the revised Sapporo criteria add value? J Rheumatology 2007;34:1522.
- Pregnolato F, Chighizola CB, Encabo S. et al. "Anti-phosphatidylserine/prothrombin antibodies: an additional diagnostic marker for APS?" Immunol Res 2013;56:432.
- Bertolaccini ML, Amengual O, Atsumi T, et al. ‘Non-criteria’ aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus 2011;20:191.
- Miyakis S, Lockshin MD, Atsumi T, et al. "International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS)". J Thromb Haemost 2006;4:295.
- Gardner C, Hills J, Machin SJ, et al. "Diagnosis of antiphospholipid syndrome in routine clinical practice". Lupus 2013;22:18.
- Forastiero R, Papalardo E, Morin K, et al. "Comparative evaluation of different immunoassays for the detection of antiphospholipid antibodies: report of a wet workshop during the International Congress on Antiphospholipid Antibodies". Arthritis Rheum 2010;62S:2252.



