Nam K. Tran, Ph.D., M.S., F.A.C.B., Director of Clinical Chemistry and POCT
Larissa May, M.D., M.S.P.H., M.S.H.S., Professor and Director of Emergency Department Antimicrobial Stewardship
Shelley Gillot, CLS Specialist (POCT)
Stacy Yee, CLS Specialist (POCT)
Introduction
For years, antigen-based rapid influenza diagnostic tests (RIDT) have been used to identify patients with influenza at the point of care. These RIDTs employ antibodies against proteins found on the influenza virus to generate results in less than 30 minutes.1 Despite the convenience of RIDTs, concerns have been raised by the Centers for Disease Control and Prevention (CDC) about the clinical sensitivity of these assays. False negative rates are common especially when influenza rates are high. Novel strains of influenza virus, such as the H1N1 swine flu, have been shown to be undetectable by RIDTs.1,2 Due to these concerns, the United States Food and Drug Administration (FDA) reclassified RIDTs from being a “Class I” device to a “Class II” device—requiring additional safeguards to improve overall RIDT performance.3 These new regulations will go into effect January 12, 2018 and have forced many manufacturers to discontinue production of RIDTs. Hospitals may continue to use RIDTs until their existing stockpiles are depleted, however, an RIDT alternative will necessary for the next influenza season.
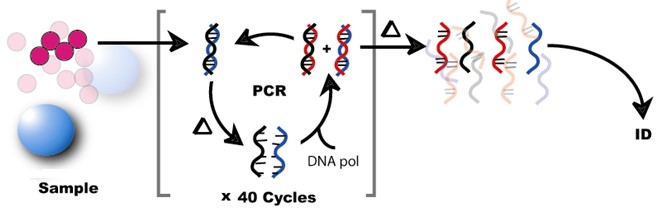
Figure 1. Conceptual Workflow for Molecular Pathogen Detection using Polymerase Chain Reaction: The figure illustrates a sample containing pathogens. Nucleic acids represented as DNA are cycled through denaturation by heat (?) (i.e. separate the standards of DNA), amplification by PCR via DNA polymerase (DNA pol), and cooled to reform doubled standard DNA steps. In the end, the nucleic acids are amplified many times and identified (ID).
Laboratory Best Practice
Molecular respiratory pathogen detection technologies have been available for nearly a decade. Many of these molecular assays employ polymerase chain reaction (PCR) to detect pathogen nucleic acids (Figure 1). However, these highly sensitive assays were labor intensive and could only be operated in the clinical laboratory environment. Interestingly, in January 2015, the first point-of-care (POC) molecular influenza A/B test was approved by the FDA—spawning a new generation of easy-to-use rapid molecular testing at the bedside.4 Rather than using antibodies against proteins found on the influenza virus (i.e., RIDTs), molecular assays target the virus' genetic make-up. In the case of influenza virus, molecular assays amplify the organism's ribonucleic acid (RNA) for detection. Using the same nasopharyngeal sample type, these molecular POC systems detect and differentiate influenza A/B in as little as 15 minutes. These POC molecular assays are not limited to influenza testing and may be employed to detect respiratory syncytial virus (RSV) Group A Streptococcus, and also Clostridium difficile. Beginning January 2018, UC Davis Medical Center will begin deployment of a PCR-based POC influenza and RSV test for the Emergency Department and our Primary Care Network.5 We discuss laboratory best practices utilizing these innovative technologies to ensure optimal patient care and test utilization:
Molecular Influenza Testing in Clinic Settings: Molecular influenza testing is not required for every patient that presents with signs and symptoms of influenza to make antiviral treatment decisions.6 Once influenza activity has been identified in the community, a clinical diagnosis of influenza can be made for outpatients with signs/symptoms consistent with suspected influenza. This workflow is very effective during periods of peak influenza activity in the community. In some cases, indiscriminate molecular influenza testing may unneccessarily increase costs and providing little to no clinical value.
Molecular Influenza Testing in Pregnant Women: The American College of Obstetrics and Gynecology (ACOG) and Society for Fetal Medicine recommends empiric treatment with antiviral therapy following CDC guidelines for pregnant women presenting with influenza-like illness.7 Molecular influenza testing is not required in this population. Molecular influenza testing could be wasteful or lead to treatment delays.
Molecular Influenza Testing in Children: The influenza infection rate is higher among infants and children, and consequently, influenza-related complications are also higher in this high-risk population. To this end, children may benefit from rapid molecular influenza testing. However, caution is advised since other respiratory diseases, such as pertussis (“whooping Cough”) may be present. It is highly recommended that at-risk children should be evaluated for other pathogens using molecular respiratory viral/bacterial panels available at UC Davis Medical Center. In the Sacramento area, pertussis infection rates have increased seven-fold from 2009 to 2010, therefore, it is recommended to include molecular testing for this pathogen in at risk children.8
Environmental Contamination: The high sensitivity of molecular assays is a “double-edged sword”. It is true that these assays outperform many RIDTs, however, there is an increased risk for false positive results.9 Unclean operating environments, poor specimen collection techniques and hand hygiene may lead to false positives. It is recommended that molecular POC operators rigorously maintain environmental cleanliness and follow manufacturer instructions for sample collection and testing.
References
- World Health Organization website: http://apps.who.int/iris/bitstream/10665/44304/1/ 9789241599283_eng.pdf, Accessed on November 1, 2017.
- Center for Disease Control and Prevention website: https://www.cdc.gov/h1n1flu/ guidance/rapid_testing.htm, Accessed on November 1, 2017.
- United States Food and Drug Administration website: https://www.fda.gov /downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MicrobiologyDevicesPanel/UCM517283.pdf, Accessed on November 1, 2017.
- United States Food and Drug Administration website: https://www.fda.gov/ NewsEvents/Newsroom/PressAnnouncements/ucm429127.htm, Accessed on November 1, 2017.
- Binnicker MJ, Epsy MJ, Irish CL, et al. Direct detection of influenza A and B virus in less than 20 minutes using a commercially available rapid PCR assay. J Clin Microbiol 2015;53:2353-2354.
- Center for Disease Control and Prevention website: https://www.cdc.gov/flu/professionals/ diagnosis/molecular-assays.htm, Accessed on November 2, 2017.
- American Association for Obstetrics and Gynecology Immunization Website: http://www.immunizationforwomen.org/, Accessed on November 2, 2017.
- Sacramento County Department of Public Health website: http://www.dhhs.saccounty.net /PUB/Pages/AZ-Health-Info/Pertussis-Whooping-Cough-Update.aspx, Accessed on November 10, 2017.
- John A, and Price CP. Existing and emerging technologies for point-of-care testing. Clin Biochem Rev 2014;35:155-167.



