Nam K. Tran, PhD, MS, FACB, Associate Clinical Professor and Director of Clinical Chemistry
Jeong Choi, MD, Fellow, Division of Cardiovascular Medicine
Jessica Rogers, MD, Resident, Dept. of Pathology and Laboratory Medicine
Jeffrey Southard, MD, Associate Clinical Professor, Division of Cardiovascular Medicine
Jennifer Jeffries, CLS, Send Outs Supervisor, Dept. of Pathology and Laboratory Medicine
Introduction
Over 5 million people in the United States have heart failure (HF).1,2 This number is expected to reach 8 million by 2030. Twenty-seven percent of Medicare patients hospitalized with HF are re-hospitalized within 30 days of discharge. The annual cost of HF in United States is reported to be $30.7 billion. Despite this growing healthcare and economic burden, laboratory tests for HF has mainly revolved around natriuretic peptides.3 Natriuretic peptide biomarkers serve as indicators of cardiac stress (i.e., biomarker of myocardial wall tension determined by chamber size and pressure) and help promote natriuresis. Brain-type natriuretic peptide (BNP) is the most common natriuretic peptide used for detecting and monitoring HF and is naturally degraded by the enzyme neprilysin in vivo (half-life = 20 mins) (Figure 1).
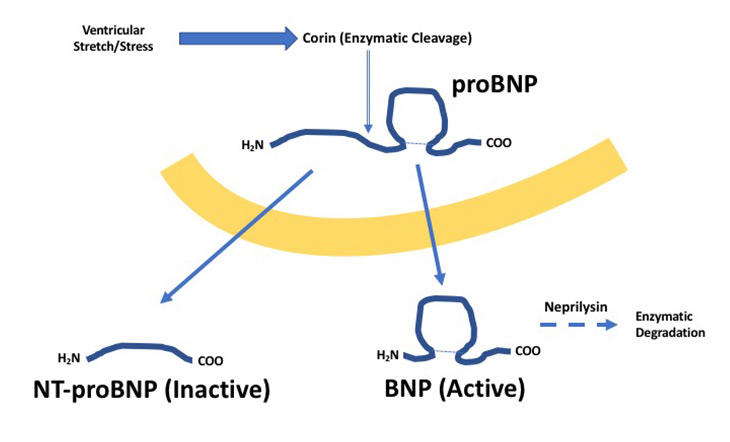
Limitations of BNP
Unfortunately, BNP carries several limitations such as significant biological variability, short half-life, and lack of assay standardization.3-5 BNP is known to have high biological variability—making serial monitoring difficult. The short half-life of BNP may result in falsely low values if specimens are not tested quickly, while the lack of assay standardization among manufacturers limits the ability to compare results from one hospital to the next. Furthermore, a new concern has been raised in patients receiving combined sacubitril/valsartan therapy.6 Sacubitril is neprilysin inhibitor which causes BNP values to remain elevated for up to 8 months following initiation of treatment. To this end, BNP may not be appropriate for HF monitoring in patients receiving sacubitril/valsartan therapy.
Lab Best Practices
Since the discovery of BNP in 1988, several other HF have emerged. These include N-terminal (NT) proBNP, soluble ST2, and galectin-3.3,7,8 We discuss these biomarkers below:
NT-proBNP: NT-proBNP is a byproduct of BNP metabolism.3-5 As shown in Figure 1, following ventricular stretching, proBNP is cleaved by the enzyme corin to produce NT-proBNP and BNP. NT-proBNP has no biological activity compared to BNP and is cleared renally—exhibiting a half-life of about 2 hours. Pharmacologically, sacubitril should not impact NT-proBNP levels. However, some studies suggest sacubitril does indirectly increase NT-proBNP glycosylation rates and influencing laboratory testing. This phenomenon is due to glycosylated forms of NT-proBNP being not measureable by clinical immunoassays. In the end, NT-proBNP decreases during sacubitril and serves as a helpful indicator of drug activity.5,6 Another advantage of NT-proBNP is its longer half-life which provides greater biomarker stability for more accurate results. Outside of sacubitril/valsartan treated patients, both BNP and NT-proBNP exhibit similar clinical performance. However, one additional benefit with using NT-proBNP is its standardization due being exclusive to one manufacturer. UC Davis Medical Center (UCDMC) will switch to NT-proBNP in 2019.
Biomarkers of Cardiac Remodeling:
The 2017 focused update of the 2013 American Heart Association Guidelines for the Management of HF includes the use of biomarkers of myocardial fibrosis.9 Two currently United States Food and Drug Administration (FDA) biomarkers are: (a) soluble ST2 and (b) galectin-3.
ST2 is part of the interleukin (IL)-1 family of receptors.7 IL-33, a cytokine known to have a cardio-protective effect, is ST2's natural ligand. In HF, fibroblasts release IL-33 to counter excessive fibrosis. Coincidentally, fibroblasts also release a soluble version of ST2 to sequester IL-33. Excessive release of soluble ST2 mitigates the cardioprotective effects of IL-33 and favoring fibrosis and remodeling. Recent studies suggest changes in soluble ST2 are more distinct than NT-proBNP, BNP, and galectin-3 and is highly predictive of cellular death, tissue fibrosis, reduced cardiac function, and an increase in the rate of disease progression. In the HF-ACTION heart failure study, soluble ST2 was a significant predictor of mortality, all-cause hospitalization, mortality due to cardiovascular disease, and hospitalization due to cardiovascular disease.9 In addition, mortality risk was significantly higher in patients with soluble ST2 values >35 ng/mL. Patients with high natriuretic peptide and soluble ST2 levels have poor prognosis. More recent studies also suggest soluble ST2 may have value for aiding the risk stratification of patients with aortic stenosis undergoing valve replacement.10 Soluble ST2 is now orderable as a Laboratory Miscellaneous Test at UCDMC.
Galectin-3 is a member of the beta-galactoside binding lectin family.8 This molecule is implicated in biological processes important to the development and progression of HF—especially progressive fibrosis and cardiac remodeling. Galectin-3 is released by macrophages involved with the inflammatory and wound healing processes following myocardial infarction. Studies suggest galectin-3 mediated HF to be highly progressive with values above 17.8 ng/mL predictive for re-hospitalization and death. Patients with high natriuretic peptide and galectin-3 levels have the worst prognosis. Galactin-3 testing at UCDMC is not supported.11
In summary, soluble ST2 and galectin-3 serve as adjuncts to natriuretic peptides. Natriuretic peptides evaluate cardiac stress, while soluble ST2 and galectin-3 are measures of cardiac fibrosis/remodeling.
Other Biomarkers: In addition to natriuretic peptides, soluble ST2, and galectin-3, other emerging biomarkers are on the horizon. It was recently reported that all detectable levels of cardiac troponin are associated with negative outcomes.12 Contemporary cardiac troponin assays cannot measure values in the majority of healthy individuals. Newer high sensitivity troponin assays can measure values in >50% of the health population—providing means to quantify cardiac troponin as low as 1 ng/L in some cases. UC Davis will switch to a high sensitivity troponin T assay in mid- to late 2018 and will be one of the first institutions using this next generation test.
References
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 Update: A report from the American Heart Association. 2014;129:e28-e292
- Jencks SF, Williams MV, and Coleman EA. Rehospitalizations among patients in the Medicare Fee-for-Service Program. N Engl J Med 2009;360:1418-1428.
- Maalouf R and Bailey S. A review of B-type natriuretic peptide monitoring: assays and biosensors. Heart Fail Rev 2016;21:567-578.
- Wu A and Smith A. Biological variation of the natriuretic peptides and their role in monitoring patients with heart failure. Eur J Heart Fail 2004;15:355-358.
- Semenov AG, Tamm NN, Apple FS, et al. Searching for a BNP standard: glycosylated proBNP as a common calibrator enables improved comparability of commercial BNP immunoassays. Clin Biochem 2017;50:181-185.
- McMurray JJ, Paker M, Desai AS, et al. Angiotension-neprilysin inhibitor versus enalapril in Heart Failure. N Engl J Med 2014;371:993-1004.
- Dieplinger B and Mueller T. Soluble ST2 in heart failure. Clin Chim Acta 2015;30:57-70.
- Sharma UC, Pokharel S, Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophined hearts and contributes to cardiac dysfunction. Circulation 2004;110:3121-3128.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail 2017;23:628-651.
- Lindman BR, Breyley JG, Schilling JD, et al. Prognostic utility of novel biomarkers of cardiovascular stress in patients with aortic stensosis undergoing valve replacement. Heart 2015;101:1382-1388.
- McCullough PA. Practical experience using galectin-3 in heart failure. Clin Chem Lab Med 2014;52:1425-1431.
- Roos A, Bandestein N, Lundback M, et al. Stable high-sensitivity cardiac troponin T levels and outcomes in patients with chest pain. J Am Coll Cardiol 2017;70:2226-2236.



