Nam K. Tran, Ph.D.1; Larissa May, M.D.2; Allen Hall, M.D.3; Tom Bullen, M.D.3;
Sarah Waldman, M.D.4; John Rodrigo, M.D.1; Julia Loegering, B.S.1; Shelley Gillott, C.L.S.1;
Stacy Yee, CLS1; Taylor Howard, MD1
1Dept. of Pathology and Laboratory Medicine; 2Dept. of Emergency Medicine; 3UC Davis Health Community Physicians; and 4Division of Infectious Diseases, Dept. of Internal Medicine
Introduction
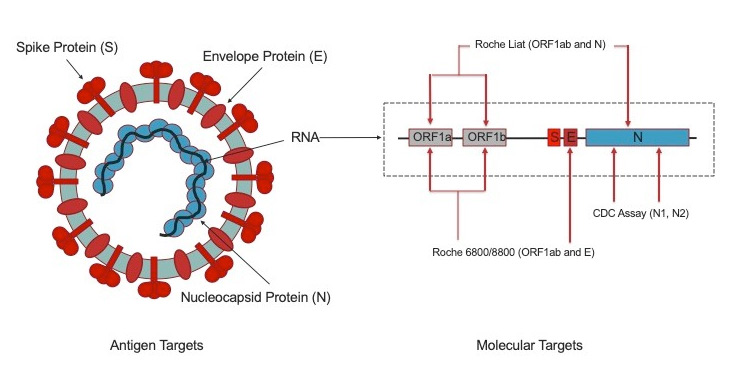
The primary means for diagnosing SARS-CoV-2 infection is by reverse transcriptase (RT) real-time polymerase chain reaction (PCR). Tests used early in the novel coronavirus (COVID-19) pandemic were based on the Centers for Disease Control and Prevention (CDC) assay targeting several regions within the SARS-CoV-2 nucleoprotein (N) gene.1 High throughput tests, including those deployed at UC Davis Health (cobas® 6800, Roche Diagnostics, Indianapolis, IN) targeted open reading frame (ORF) 1ab and the envelope protein (E) genes (Figure 1).2 Unfortunately, these early RT-PCR tests used “batched” testing schemes (e.g., needing to test multiple samples at one time) to optimize reagent use, and required operation by licensed high complexity laboratory personnel, and exhibiting testing turnaround times that were not compatible with “STAT” (<1 hour) needs.
Point-of-care (POC) SARS-CoV-2 molecular testing solutions emerged March 27, 2020. One POC platform relied on isothermal nucleic acid amplification techniques. However, these early POC tests exhibited less than ideal clinical sensitivity due to their isothermal detection methods.3 To this end, for most of the COVID-19 pandemic, the world lacked a viable high sensitivity rapid POC RT-PCR solution to accelerate decision making in at risk populations and facilitate patient isolation, and contact tracing.
On November 3, 2020, UC Davis Health deployed the first commercially available high sensitivity rapid POC PCR platform (cobas® Liat, Roche Diagnostics, Indianapolis, IN) for detecting SARS-CoV-2 and also differentiating between influenza A and B viruses. The test will be rolled out in the Emergency Department and select clinics within the health system. Testing can be performed by Clinical Laboratory Improvement Amendment (CLIA) waived users including physicians and nurses. The primary specimen type will be nasopharyngeal (NP) swabs at this time. For SARS-CoV-2 detection, this assay targets regions in ORF1ab and N genes (Figure 1)4 which exhibits clinical sensitivity and specificity of >99.9% with 100% agreement with to our high through / high sensitivity cobas 6800 laboratory-based PCR method. Internal validation at UC Davis Health has found the cobas Liat sensitivity to remain comparable to the cobas 6800 even with low viral load samples. This rapid POC PCR platform has been used since early 2018 for influenza A/B, respiratory syncytial virus (RSV), and Group A Streptococcus testing and will now add SARS-CoV-2 testing capability to the emergency department and ambulatory care settings. Implementation of this rapid POC PCR platform continues the Department of Pathology and Laboratory Medicine's COVID-19 testing strategy (established on February 29), to first rapidly deploying initial SARS-CoV-2 RNA testing capability (March 19), then scale testing capacity to meet health system needs (March 28), and now further accelerate turnaround times for special patient populations. The new POC platform complements our existing rapid random access (2 hour) test (ePlex, GenMark, Carlsbad, CA) deployed on April 30 for use with patients undergoing urgent continuous aerosol generating procedures (AGP), emergency patients in respiratory distress, or deceased donor kidney transplant recipients.
Laboratory Best Practice
Not all patients require a rapid 20-minute SARS-CoV-2 PCR test. UC Davis Health is fortunate to have several testing options for our patients, healthcare workers, and community—often providing results in less than 24 hours. The preferred specimen type remains the NP swab. Saliva is not approved on any UCDH platform at this time due to variable sensitivity and not all patients may produce adequate saliva for testing. Other samples types are under evaluation for future deployment.
Due to the high sensitivity and specificity of the new rapid combined influenza A/B and SARS-CoV-2 POC RT-PCR test, confirmation of POC results with a laboratory method is discouraged. Both UC Davis Health and the FDA data illustrate this test to be comparable to our laboratory RT-PCR assays.
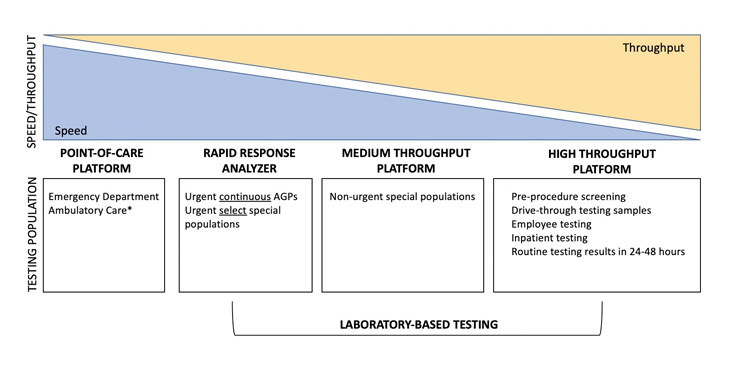
With influenza season approaching, the rapid POC RT-PCR test should also be used when results facilitate timely influenza anti-viral treatment and support personal protective equipment decisions. Testing is recommended for patients who are very sick with/without respiratory distress, needing a rapid result to return to congregate living facilities, and/or support urgent contact tracing and isolation needs (e.g., identifying hospital patient/employee COVID-19 clusters, etc). If patients do not meet these testing requirements, it is encouraged to leverage the laboratory-based high throughput SARS-CoV-2 assay. Urgent continuous AGPs or deceased donor kidney transplant cases should request the 2-hour rapid test performed in the laboratory. Figure 2 conceptually illustrates the speed versus throughput tradeoff for various platforms and their recommended testing populations at UC Davis Health.
Not all SARS-CoV-2 tests are created equal including molecular tests. All UC Davis Health platforms were selected due to their ability to provide highly sensitive and specific testing performance. For molecular testing in particular, assays with <95% sensitivity should be discouraged due to unacceptable false negative rates. The same applies for non-molecular tests. Non-molecular tests, such as antigen assays, are not presently being pursued clinically at UC Davis Health at this time, although research studies are ongoing. In contrast to molecular tests, antigen tests detect the viral proteins illustrated in Figure 1. Antigen testing remains limited to symptomatic individuals, currently exhibit lower sensitivity / specificity compared to PCR methods, and supplies have been limited due to sequestration by United States Department of Health and Human Services.5 With that said, it is anticipated that antigen testing performance will improve over time and could one day provide means to reliably screen asymptomatic or pre-symptomatic individuals and complement RT-PCR methods. However, at present, the primary means to for diagnosing SARS-CoV-2 infection remains molecular diagnostic methods such as RT-PCR.
REFERENCES
- Basu A, et al. Performance of Abbott ID Now COVID-19 rapid nucleic acid test using nasopharyngeal swabs transported in viral transport media and dry nasal swabs in a New York City academic institution. J Clin Microbiol 2020;58:e01136-20.
- CDC Flu / SARS-CoV-2 PCR test information for use document: Accessed on October 1, 2020
- Roche Diagnostics SARS-CoV-2 6800/8800 PCR test information for use document: Accessed on October 1, 2020.
- Roche Diagnostics Liat SARS-CoV-2/Flu AB PCR test information for use document: Accessed on October 1, 2020.
- United States Health and Human Services website: Accessed on October 1, 2020.



