Gopal Patel, Zarir Karanjawala, Denis M. Dwyre
The morphologic analysis of bone marrow specimen is an essential part of comprehensive evaluation in the diagnosis of hematologic and certain non-hematologic disorders. Some of the most common indications for bone marrow examination include a) unexplained cytopenias, cytosis, or abnormal blood cell morphology, suggestive of bone marrow pathology. b) diagnosis, staging, and therapeutic follow-up of hematologic malignancies, c) investigation of suspicious metastatic disease or bony lesions on imaging study, d) unexplained organomegaly, e) fever of unknown origin with suspicion for microbial infection or occult hematologic malignancy, and f) investigation of iron stores, lipid/glycogen storage disorders, or nutritional deficiencies (1). In this lab best practice blog, we will review the complementary roles of peripheral blood smear and bone marrow core biopsy and aspirate in the diagnosis of various hematologic malignancies.
1) Peripheral Blood Smear
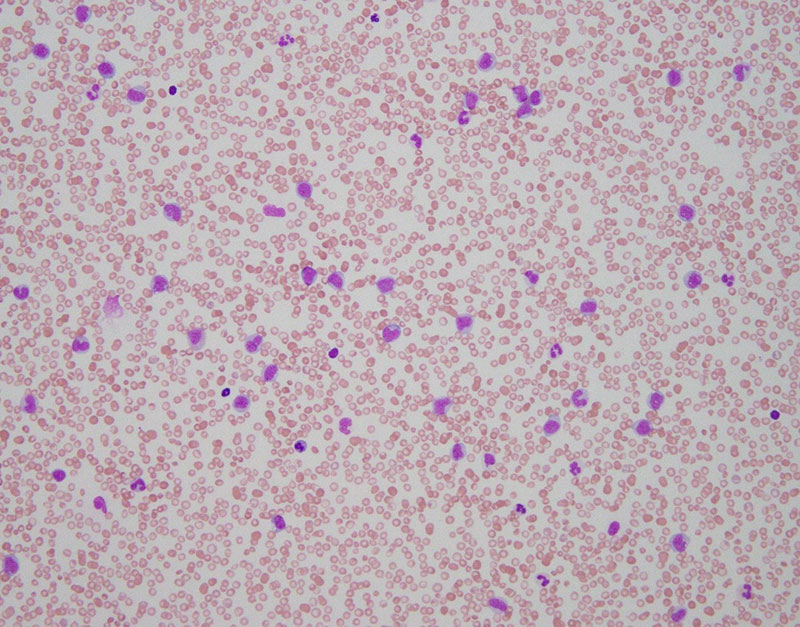
Although patients with hematologic diseases may present with various sign and symptoms, an abnormal complete blood count (CBC) during routine medical visits is a common indication that prompts further investigation and eventual bone marrow evaluation. Therefore, examination of CBC data and peripheral blood smears are the first diagnostic step in defining a hematologic disease. Peripheral blood often provides clues to the underlying bone marrow pathology (Fig. 1). For example, when myelodysplastic syndrome (a bone marrow failure disorder) is suspected, a well stained peripheral blood smear is very important for the evaluation of cytopenias. Dysplastic neutrophils (hypolobated nuclei, hypogranular cytoplasm, etc.) and platelet size and granularity are best evaluated in peripheral blood smears. In cases of suspected myelofibrosis, tear-drop cells and/or leukoerythroblastic changes are often seen in peripheral blood smears. Some suspected hematopoietic malignancies are evaluated based on routine peripheral blood data in asymptomatic patients and bone marrow work-up is performed to determine the extent and/or progression of disease. For example, chronic lymphocytic leukemia is defined by the presence of monoclonal B-cell population, with characteristic morphology and immunophenotype, in peripheral circulation. WHO 2016 recommends morphologic evaluation and manual 200 leukocyte differential counts in peripheral blood smear as part of complete bone marrow evaluation (2). According to a recent study, a peripheral blood smear review performed for the assessment of abnormal cell morphology, hematolymphoid neoplasm, leukocytosis, circulating blasts, and parasitic infection has significant added clinical value (3).
2) Bone marrow evaluation
A bone marrow evaluation is called for when available clinical and laboratory results cannot adequately explain the hematological abnormalities. Generally, a thorough bone marrow evaluation may include trephine bone marrow core biopsy, touch imprints of a bone marrow core biopsy, aspirate smears, spicule crush, and aspirate clot preparation (4). In addition, a bone marrow investigation invariantly includes some of the ancillary studies, such as special stains, cytochemistry, immunohistochemical studies, immunophenotypic analysis (flow cytometry), cytogenetics, molecular genetics, and other specialized investigations.
The International Council for Standardization in Hematology (ICSH) has prepared a set of guidelines based on preferred best practices that is widely utilized and accepted for the collection and reporting of bone marrow specimen (1). Together the aspirate and trephine biopsy provide a complementary and comprehensive evaluation of the bone marrow. The final bone marrow report requires the integration of peripheral blood findings, bone marrow core and aspirate findings, evaluated together with the results of supplementary tests such as cytogenetics, immunophenotyping, and molecular genetics studies. Therefore, most authorities advocate obtaining both the aspirate and trephine biopsy, and clinicians must attempt to collect both for an optimal bone marrow evaluation.
The sequence of obtaining marrow specimen is controversial. Collection of core biopsy first releases thromboplastic elements that can cause clotting and compromise the quality of aspiration. Procurement of aspirate first cause aspiration artifacts in core biopsy (hypocellularity, sinusoidal hemorrhage, etc.). Nonetheless, the sequence is unimportant if both specimens are obtained as part of the same procedure. Many different techniques and biopsy equipment exist that have greatly facilitated bone marrow sampling with minimal artifacts and distortions (5).
2a) Bone marrow trephine biopsy
Trephination is the oldest surgical procedure for which archaeological evidence exists (6). Its use for pathologic bone marrow sampling was first described in 1903 by G. Pianese (7). Current guidelines from WHO recommends collection of ≥ 1.5 cm long bone marrow core with ≥ 10 partially preserved intertrabecular areas.
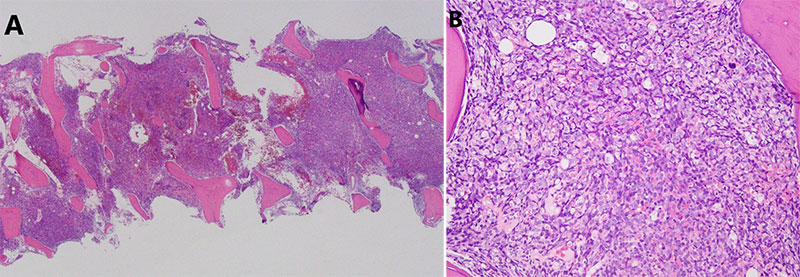
Bone marrow core biopsy can be a painful process for patients. Additionally, biopsy processing takes a longer time and multiple steps because it requires additional processing, namely decalcification. However, it allows in situ morphologic and immunophenotypic analysis that are important for accurate assessment of cellularity, architecture, infiltrative processes, and spatial relationships between hematopoietic elements (Fig. 2). Unexplained pancytopenia and leucoerythroblastic-like blood smear are indications for core bone marrow biopsy because they are likely to demonstrate marrow fibrosis (reticulin and collagen). In cases where adequate aspiration material is not obtained (i.e. fibrosis or cell packing), an additional core biopsy, after disaggregation, may provide additional cellular material for flow cytometry, cytogenetics, and molecular analysis. Core biopsy is mandatory in the cases with dry tap to determine the nature of pathologic process (7). Core biopsy is superior for evaluation of microorganisms, aplastic anemia, different phases of myeloproliferative neoplasms, plasma cell myeloma, and in tuberculosis and sarcoid granulomas (8, 9). In hematolymphoid malignancies the formation and localization of lymphoid aggregates can be demonstrated on core biopsy. Classic Hodgkin lymphoma can be evaluated on a core biopsy. Core biopsy is more useful than an aspirate in assessing amyloid deposition and for staging metastatic malignancies to the bone. Core biopsy is essential in initial staging of many pediatric non-hematopoietic malignancies, such as rhabdomyosarcoma, neuroblastoma, PNETs, and Ewing sarcoma. Many adult non-hematopoietic malignancies, such as sarcoma of the bone, also require bone marrow biopsy for staging.
2b) Bone marrow aspirate
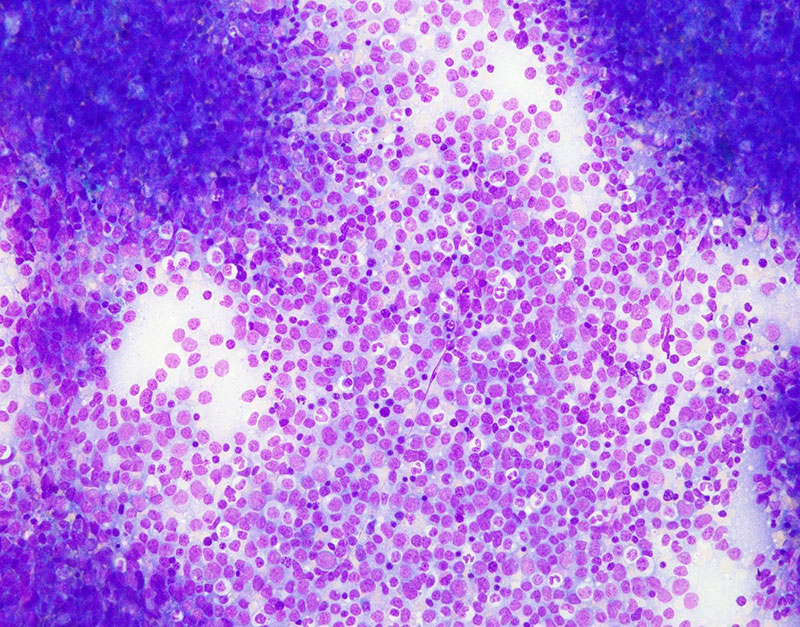
Bone marrow aspiration is a reliable and rapid method of evaluating bone marrow pathology (10). It is especially preferred in clinically urgent cases because aspirate slides are easy to make and usually can be stained with readily available stains without need for elaborate processing. The advantage of bone marrow aspirate lies in its ability to allow cytomorphologic analysis and cell enumeration (Fig. 3). Bone marrow aspirate is important in the evaluation of acute leukemias and MDS, where an accurate enumeration of the blasts is essential for diagnosis. Aspirate is also important in diseases with patchy marrow involvement (i.e. plasma cell dyscrasias). However, with the advancement in cytogenetics and molecular techniques, and the ability to incorporate such data for diagnostic and prognostic purposes, the bone marrow aspirate has become even more important. Hematolymphoid malignancies can evolve and change both morphologically and immunophenotypically as diseases progress due to accumulation of additional mutation over time. Therefore, molecular analysis is the best tool to follow up with disease progression in these patients. Unfortunately, trephine bone marrow biopsy material cannot be used for molecular study since decalcification process degrades DNA and RNA. However, aspirate and its derivatives (i.e. clot preparation) can be used for molecular studies. In WHO 2016 classification of tumors of hematopoietic and lymphoid tissues, many leukemias and lymphomas are classified based upon molecular studies. For example, the diagnosis of acute promyelocytic leukemia is based upon the molecular findings of PML-RARA fusion gene generated by the t(15; 17) chromosomal translocation. Therefore, bone marrow aspirate is important and must be collected along with trephine biopsy.
Conclusion
Bone marrow evaluation is a complex, but essential multimodal process for the diagnosis of hematologic malignancies and many non-neoplastic disorders. Every attempt should be made to collect and submit both the trephine core biopsy and bone marrow aspirate along with peripheral blood sample since they together provide the most comprehensive and complementary information in the diagnostic work-up of hematolymphoid malignancies.
References
- Lee, S.-H. et. al. (2008) ICSH guidelines for the standardization of bone marrow specimens and reports. Int. Jnl. Lab. Hem. 30, 349-364.
- Swerdlow, S. H. et. al. (Eds): WHO classification of tumors of haematopoietic and lymphoid tissues (Revised 4th ed.). IARC: Lyon 2017.
- Beckman, A. K. et. al. (2020) Clinician-ordered peripheral blood smears have low reimbursement and variable clinical value: a three-institution study, with suggestions for operational efficiency. Diagnostic Pathology. 15, 112-201.
- Bain, B. J. (2001) Bone marrow aspiration. J. Clin. Pathol. 54, 657-663.
- Islam, A. (2007) Bone marrow aspiration before bone marrow core biopsy using the same bone marrow biopsy needle: a good or bad practice? J. Clin. Pathol. 60, 212-215.
- Parapia, L. A. (2007) Trepanning or trephines: a history of bone marrow biopsy. Brit. J. Haematology. 139, 14-19.
- Riley, R. S. et. al. (2004) A pathologist’s perspective on bone marrow aspiration and biopsy: I. Performing a bone marrow examination. J. Clin. Lab. Analysis. 18, 70-90.
- Kaur, M. et. al. (2014) Diagnostic value of bone marrow aspiration and biopsy in routine hematology practice. J. Clin. Diag. Res. 8, 13-16.
- Bain, B. J. (2001) Bone marrow trephine biopsy. J. Clin. Pathol. 54, 737-742.
- Afkhami, A. et. al. Peripheral blood smears, bone marrow aspirate trephine and clot biopsies: methods and protocols, page 257-270, in Day, C. E. (Eds.): Histopathology methods and protocols. Humana Press: New York 2014.



