UC Davis developing models to predict the excitability of brain neuronal circuits
Understanding the link between the imbalance in neural electrical channels and neurodevelopment disabilities
The brain has around 86 billion interconnected neurons. The connections between these neurons form brain circuits, or paths, for electrical signaling to follow as it moves from one nerve cell to another.
“The basic function of neurons is to fire electrical action potentials, or electric signals, to transfer information. These signals are generated by the movement of charged ions across channels in the neural membrane to either excite or inhibit neuronal activity,” said Roy Ben-Shalom, an assistant professor of neurology and faculty member at the UC Davis MIND Institute.
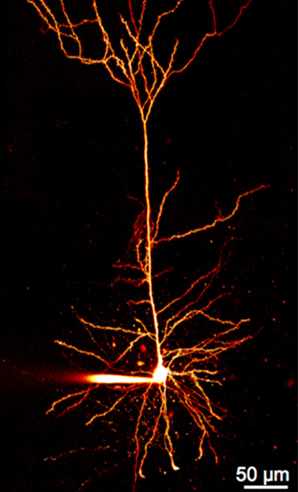
Firing action potentials is also essential for coordinating brain function. The proper timing and patterns of this neuronal activity affect all aspects of cognition, behavior and nervous system function. Channelopathies, or mutations in ion channels, can disrupt the balance between excitatory and inhibitory neuronal activation. They lead to impaired brain connectivity and abnormal neuronal activity patterns.
“Excitatory-inhibitory imbalance is a hallmark of many neurodevelopmental disabilities, particularly autism and childhood epilepsy. This imbalance causes disrupted neuronal circuits with abnormal connections between the neurons,” Ben-Shalom explained.
He is working on predicting the excitability of brain neuronal circuits in neurodevelopmental conditions. In April, The Hartwell Foundation selected him to receive 2022 Individual Biomedical Research Award. The award funds early-stage, innovative and cutting-edge biomedical research to benefit children in the United States.
“The mechanisms by which channelopathies lead to neurodevelopmental disabilities are poorly understood,” Ben-Shalom said. “By simulating how mutations in ion channels alter neuronal activity, we can gain insights into the underlying mechanisms of these conditions and identify potential therapeutic strategies.”
With our tool, we will be able to identify which combination of drugs would optimize treatment for each patient, eliminating the trial and error process.” —Roy Ben-Shalom
Developing personalized treatments to neurodevelopmental conditions
According to Ben-Shalom, therapies for millions of children with neurodevelopmental conditions are designed by trial and error and based on clinical history. There is a need for better treatments anchored in personalized interventions that are specific to the child and the condition.
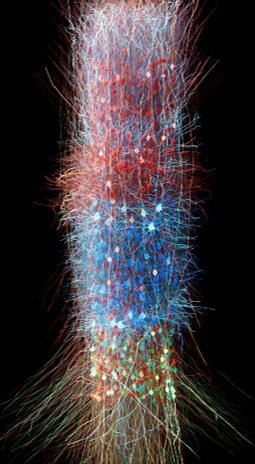
Ben-Shalom is developing a computer model that simulates changes in neuronal activity caused by alterations in ion channel function. The model will be optimized based on data from cells expressing specific channel mutations and recording the electrical signals directly from the affected channels.
“A key innovative factor of the model is in understanding how specific mutations affect electrical signaling, disrupt normal neuronal circuitry, and contribute to neurodevelopmental disabilities,” Ben-Shalom explained. “Most importantly, the model can be used to simulate the effects of treatment and inform the selection of the most effective therapy for each channel mutation.”
If successful, the simulation model will enable a better understanding of the mechanisms underlying some conditions. This will allow providers to choose specific, effective therapies to restore proper neuronal function.
“Optimizing the right treatment to the right patient is very hard and involves a lot of trial and error. We are proposing a new way to optimally treat childhood epilepsy and other neurodevelopmental conditions. With our tool, we will be able to identify which combination of drugs would optimize treatment for each patient, eliminating the trial and error process,” Ben-Shalom said.