Residency Program - Case of the Month
May 2010 Final Diagnosis - Presented by Brian Gorospe, M.D.
Answer:
Malignant Melanoma, Amelanotic type
Histological description
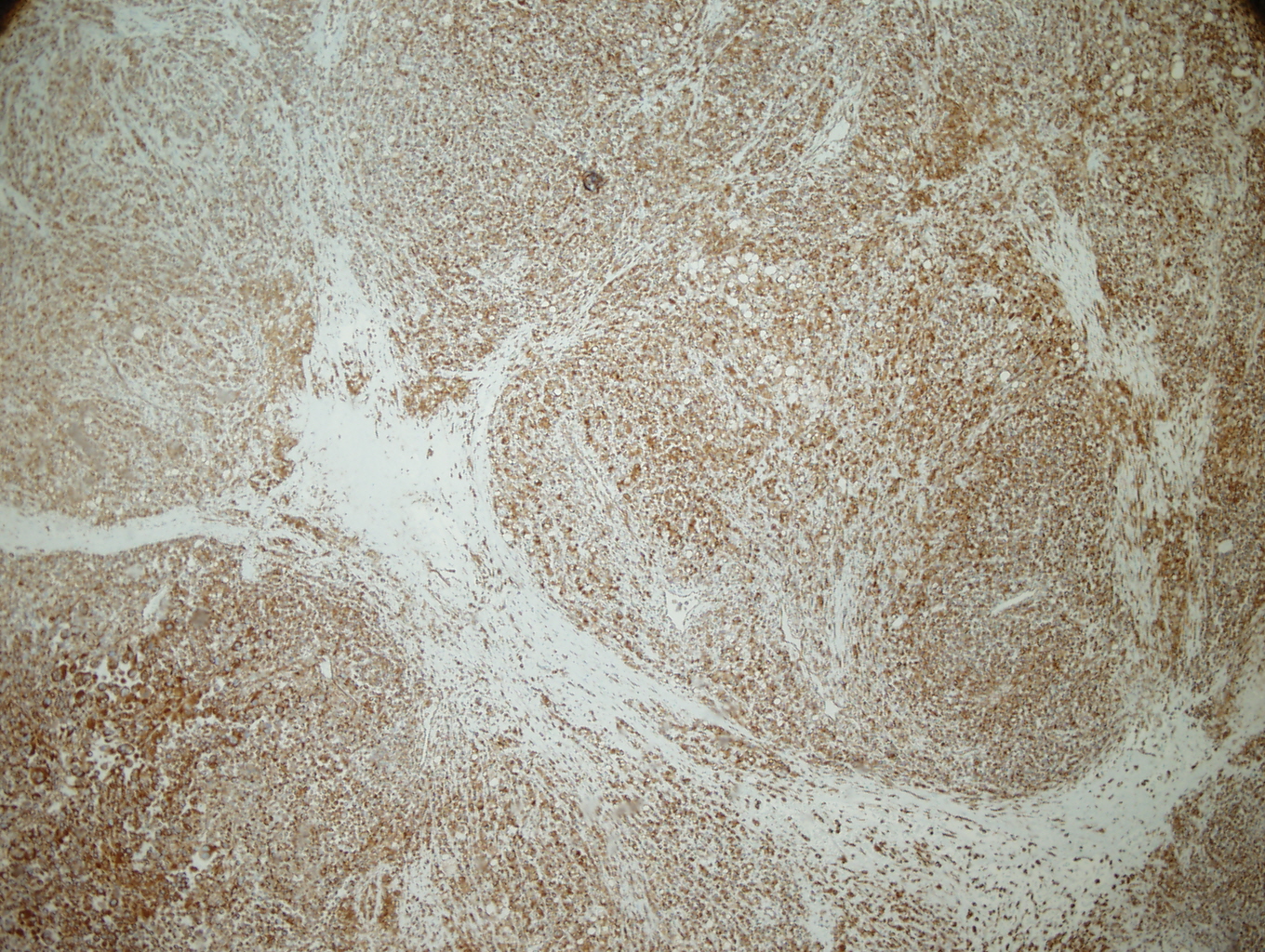
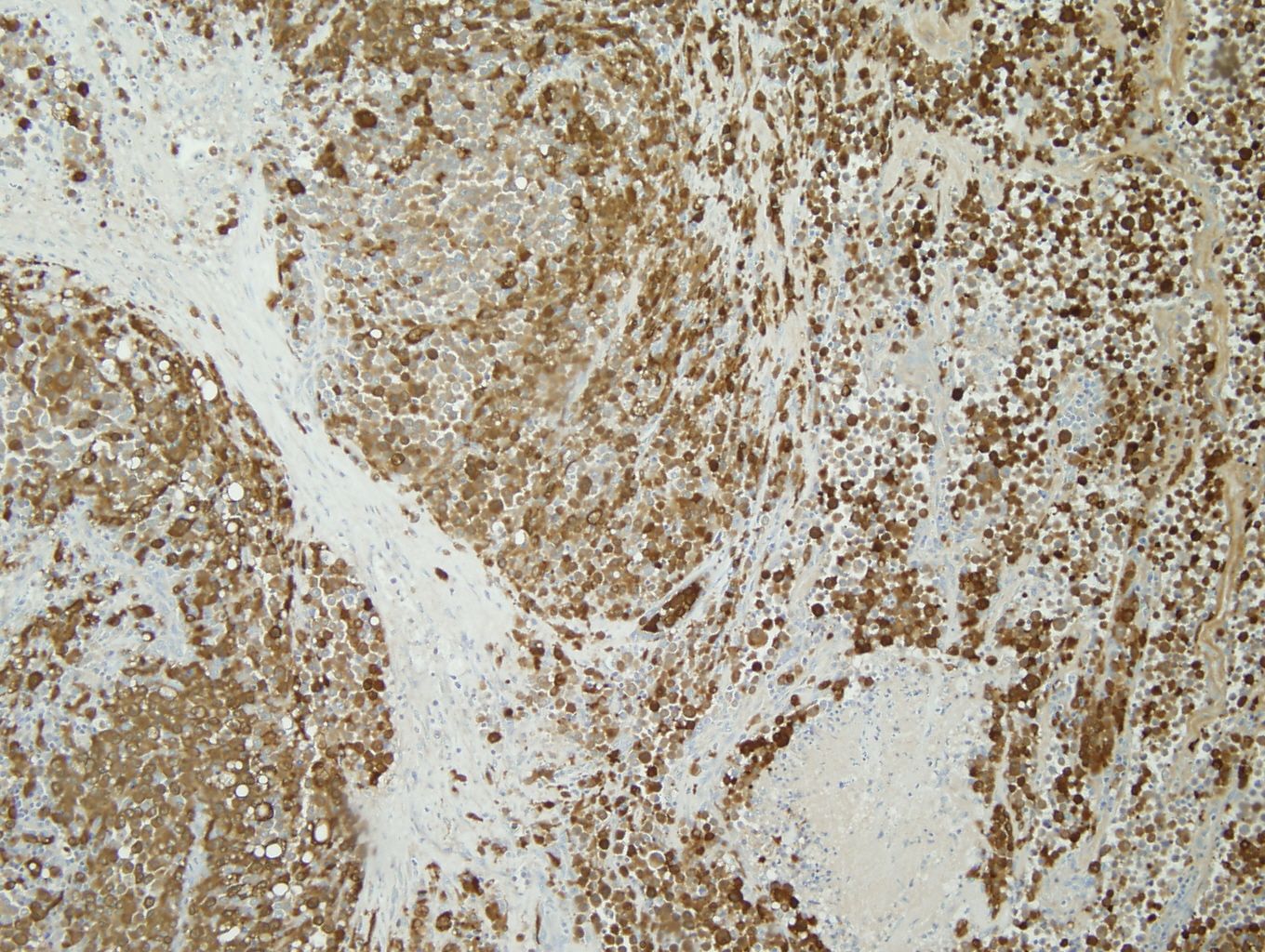
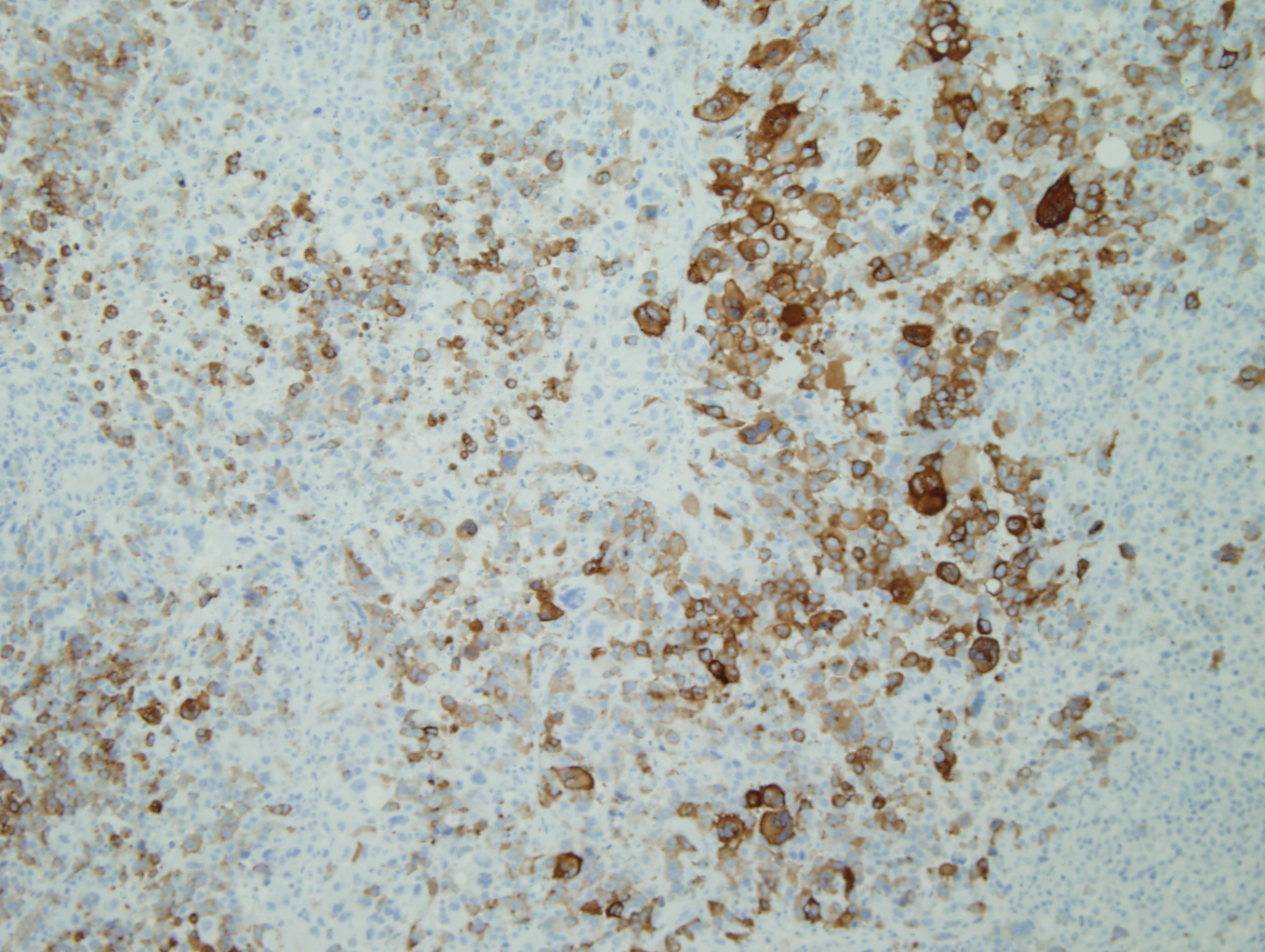
The tumor was a 9cm mass located between the upper and lower left pulmonary lobes and adjacent to the bronchus. The tumor involved the parenchyma of both the upper and lower lobes. Microscopic examination showed a poorly differentiated malignant neoplasm with solid, nested, and alveolar growth patterns, and areas of necrosis.The tumor cells had a wide variety of appearances, predominantly epitheliod, with some spindle cells and clear cells. Scattered individual large multinucleated malignant cells were noted in the background. The tumor extended into the peribronchial/hilar lymph nodes. Lymphovascular invasion was identified. There was no direct invasion of the bronchial epithelium identified. The remaining lung parenchyma was unremarkable. The malignant cells were positive for Vimentin, S-100, HMB-45, and Melan-A. The malignant cells were negative for cytokeratins, CD 21, CD23, CD31, CD35, CD45, CD68, CD117, HMA, Desmin, OCT4, PLAP, Synaptophysin, and Chromgranin.
Immunohistochemistry
|
Figure 1: Vimentin |
Figure 2: S-100 |
|
Figure 3: HMB-45 |
Figure 4: Melan-A |
Discussion:
The negative staining of the malignant cells for cytokeratins and positive staining for S-100, HMB-45, and Melan-A strongly supported the diagnosis of malignant melanoma. The tumor may have represented either a primary malignant melanoma of the lung (PMML) or metastatic disease to the lung. A thorough review of the patient's past medical history and a complete clinical evaluation failed to reveal a history of, or the presence of any suspicious skin or mucosal lesions. Additionally, further body imaging (PET-CT, brain MRI) revealed no other masses or areas of possible malignancy or metastatic disease. The lack of a primarily melanoma elsewhere in this patient supported the diagnosis of PMML. However, the diagnosis of PMML could not be made with absolute certainty due to the well-documented capacity for spontaneous regression of primary melanomas in cutaneous and mucosal sites. (1)
Melanoma is a malignant tumor derived from melanocytes and is the most common form of fatal skin neoplasm. Though it most commonly occurs in sun-exposed areas of skin, their occasional origin in noncutaneous sites is well documented in the literature. Ocular and conjuctival melanomas are the most common noncutaneous sites, but it also well recognized to arise in the nasopharynx, oropharynx, the leptomeninges, the adrenal gland, the urethra, the vulva, the ovaries, the vagina, and the gastrointestinal tract. (2, 3)
PMML is a rare tumor, with fewer than 30 cases reported in the literature. The ages of reported patients with PMML ranges from 29 to 80 years (median 51 years), with an equal sex distribution. Patients with PMML generally present with symptoms such cough, hemoptysis, or post obstructive pneumonia. (1,2,4)
The diagnosis of PMML is controversial due of the difficulty in excluding metastatic disease from a silent or regressed primary. Metastatic melanoma to the lungs is common and may present a solitary mass-like lesion. Due to this difficulty in differentiating metastatic versus PMML, both clinical and histologic criteria have been proposed to exclude extrapulmonary origin. Jensen and Egerdorf proposed clinical criteria for the diagnosis of PMML requiring that there be no history suggestive of a previous melanoma (cutaneous or ocular); no demonstrable melanoma of any other organ at the time of diagnosis; the presence of a solitary, central tumor of the lung; tumor morphology compatible with a primary malignant tumor by immunohistochemistry and/or electron microscopy; and no evidence at autopsy compatible with a primary melanoma elsewhere. (3) Histologic requirements were proposed by Allen and Drash and include the identification of a bronchial intraepithelial component or invasive nevus in association with the invasive component of the tumor. (2,5) However it should be noted that absence of this criteria should not necessarily exclude the diagnosis of PMML, as the in situ component may be absent due to overgrowth of the invasive component and ulceration of the bronchial epithelium. (5)
As melanocytes have not been demonstrated in the normal tracheobronchial tree, several theories on the histogenesis of PMML have been suggested. One theory involves the migration of melanocytes to the tracheobronchial tree during embryogenesis. Melanocytes have been identified in the mucosa of the oropharynx, the larynx, and the esophagus. Because the bronchi and lungs are embryologically so closely related to the oropharynx, larynx, and esophagus, and of similar endodermal derivation, it is thought that melanocytes may migrate to the bronchial mucosa and be present on rare occasions. Alternate theories of histogenesis propose that the tumor cells might arise from a process of "melanogenic metaplasia" occurring in the submucosal bronchial glands. While others hypothesize that the tumor cells might arise from precursor cells that have the potential to undergo melanocytic differentiation. (3,5)
Architecturally and cytologically, the tumor cells of PMML are similar to melanomas of other sites and keep with melanoma's reputation as "the great imitator". (3,4) In one of the largest series of cases that met criteria for PMML, completed by AFIP, the histologic growth pattern varied from organoid to fasicular and included epitheloid to spindled cells with hyperchromatic to vesicular nuclei, prominent eosinophilic nucleoli, and abundant eosinophilic to clear cytoplasm with occasional intranuclear cytoplasmic inclusions. (5) Electron microscopy can aid with the diagnosis of PMML by confirming the presence of premelanosomes. (1) However, in cases of amelanotic melanomas (such as the case described), premelanosomes may be absent. (6) Immunohistochemical negativity for keratin and positivity for S-100, HMB-45, Melan-A are useful in confirming the presence of melanocytic differentiation and excluding of other diagnoses in the differential. (1)
In addition to metastatic malignant melanoma to the lung, the primary differential diagnoses for PMML include carcinoid tumor (especially melanotic carcinoid). Carcinoid cases can be differentiated by their keratin and neuroendocrine marker positivity, as well as, their more uniform cytologic features. Large cell carcinomas may also be included in the differential for amelanotic PMML, but should be positive for cytokeratin and negative for S-100 and HMB-45. (4) Other rare tumors that show similar staining for melanoma markers include sugar tumors and metastatic clear cell carcinoma. Sugar tumors should show additional reactivity with CD117, Myo-D1, or actin. Metastatic clear cell sarcoma typically also have a characteristic chromosomal t(12;22)(q13;q12) translocation involving the Ewing sarcoma gene (EWSR1). (1,7) FISH studies on tissue from the case described did not show rearrangement of EWSR1 locus, but instead demonstrated the BRAF gene mutation, which is present in approximately 70% of melanomas.
The treatment of PMML is aggressive surgical resection, usually involving a lobectomy or pneumonectomy. The prognosis is generally poor with the majority of patients dying within one year of diagnosis. However, patients remaining free of disease for up to 11 years after resection have been reported. (1,4)
References:
- Leslie K, Wick MR (Eds). Practical Pulmonary Pathology. Philadelphia: Churchill Livingstone, 2005.
- Ost D, Joseph C, Sogoloff H, Menezes G. Primary Pulmonary Melanoma: Case Report and Literature Review. Mayo Clin Proc 1997; 74:62-66.
- Thompson JF, Bishop JF. 1999. Primary melanoma of the lung. In Raghavan D, Brecher ML, Johnson DH, Meropol NJ, Moots PL, Thigpen JT (Eds.) Textbook of Uncommon Cancer (pp537-540). New Jersey: John Wiley & Sons Ltd , 1999.
- Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (eds). Pathology and Genetics Tumors of the Lung, Pleura, Thymus, and Heart. Albany: WHO Press, 2004.
- Wilson R, Moran C. Primary Melanoma of the Lung: A Clinicopathologic and Immunohistochemical Study of Eight Cases. Amer J Surg Path 1997, 21:1196-1202.
- Erlandson R. Ultrastuctural Diagnosis of Amelanotic Malignant Melanoma: Aberrant Melanosomes, Myelin Figures, or Lysosomes? Ultrastructural Path 1987; 11:191-208.
- Weiss SW, Goldblum JR (eds). Enzinger and Weiss's Soft Tissue Tumors 5th Ed. Philadelphia: Mosby, 2008.


 Meet our Residency Program Director
Meet our Residency Program Director
