Residency Program - Case of the Month
August 2010 Final Diagnosis - Presented by Diane Sanders, M.D.
Answer:
Medullary Thyroid Carcinoma (MTC)
Microscopic description
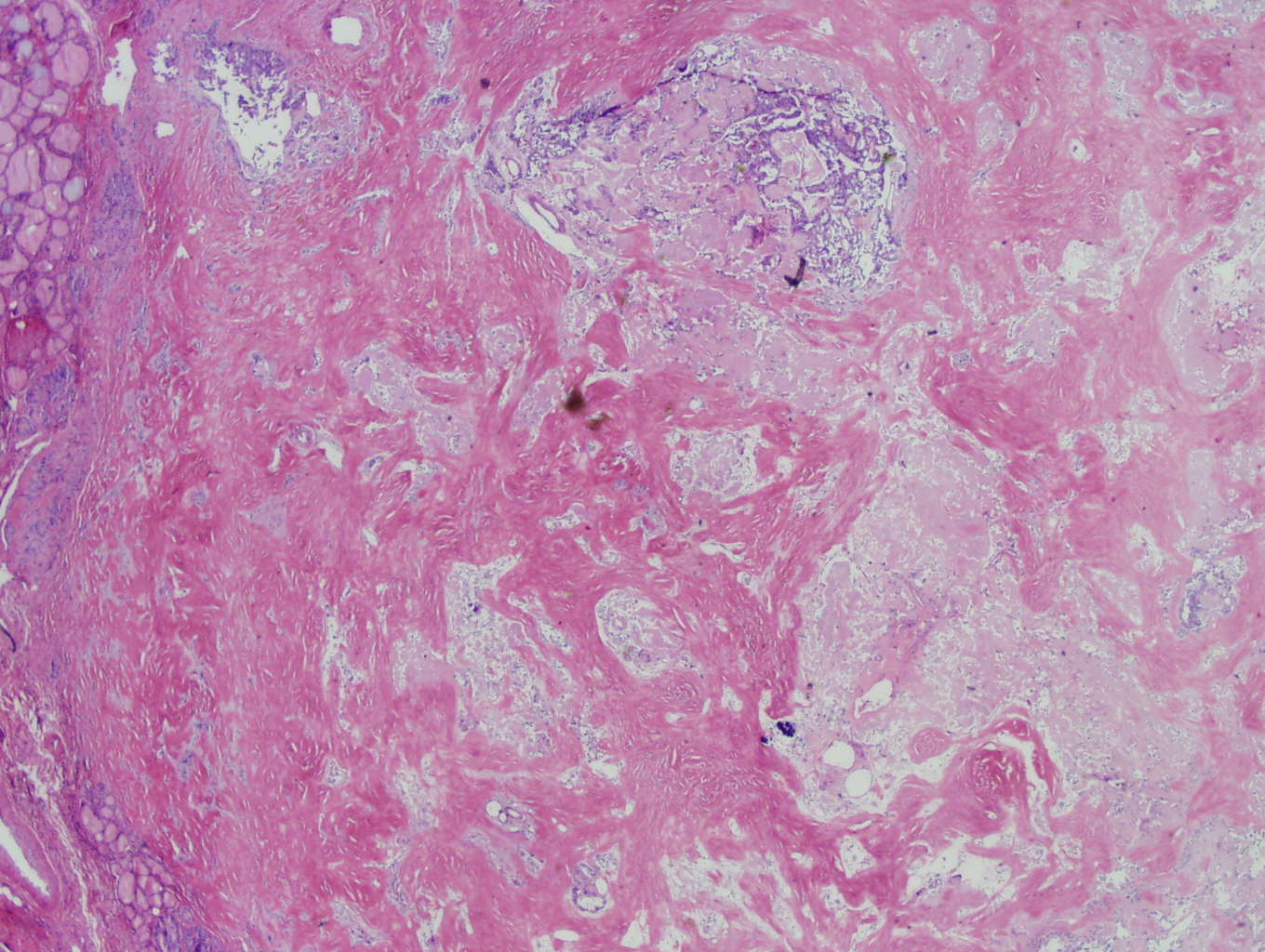
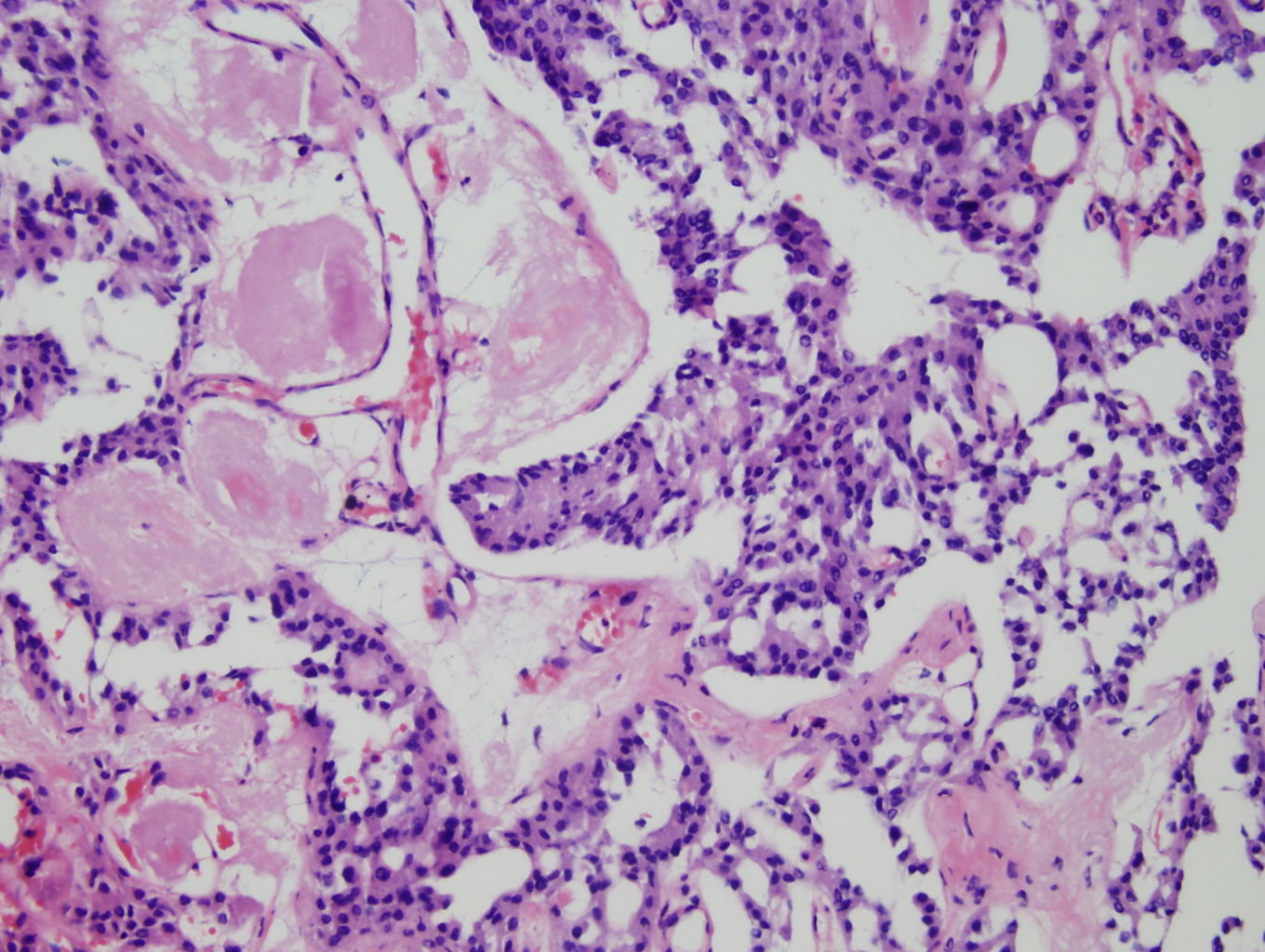
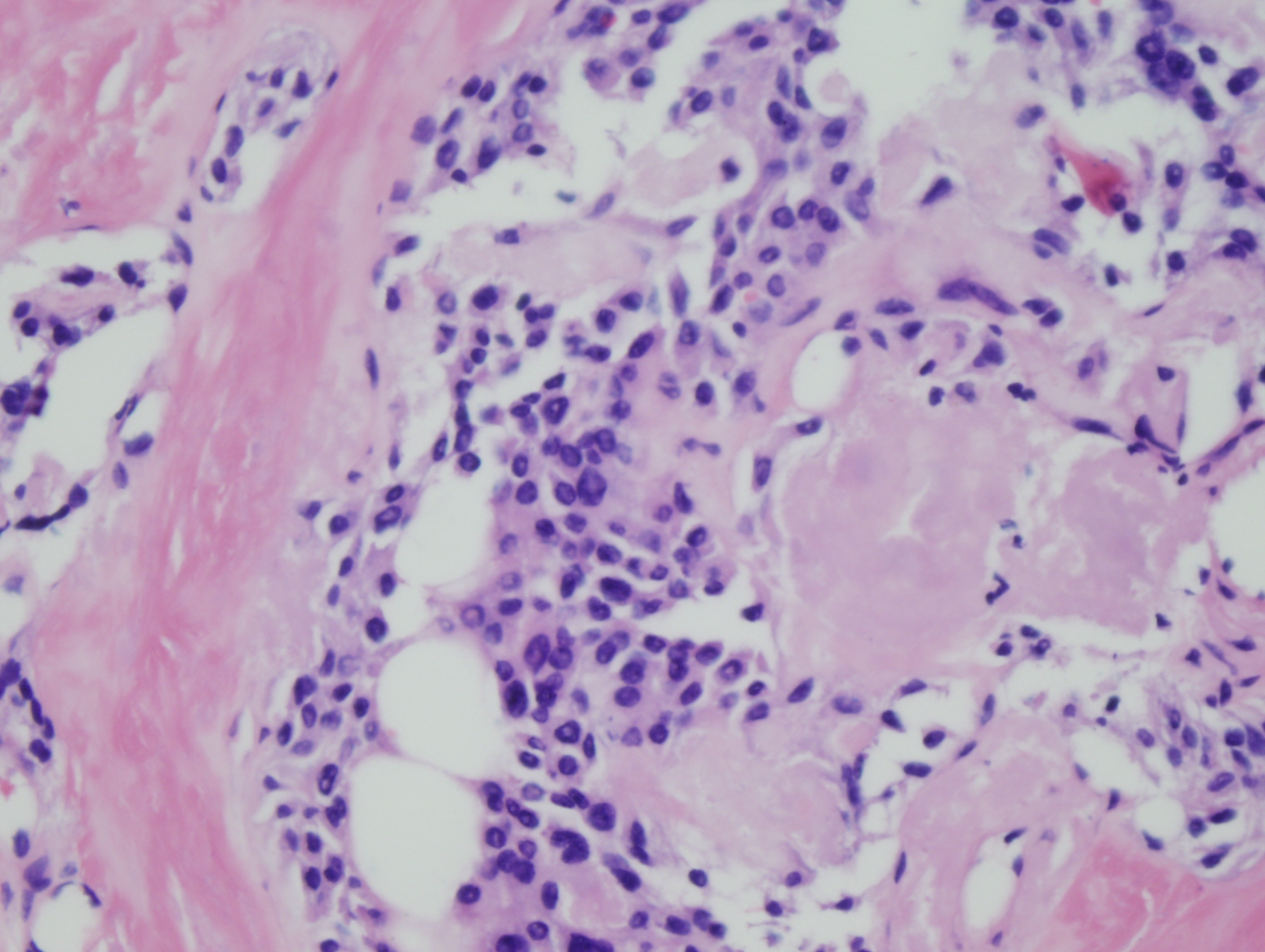
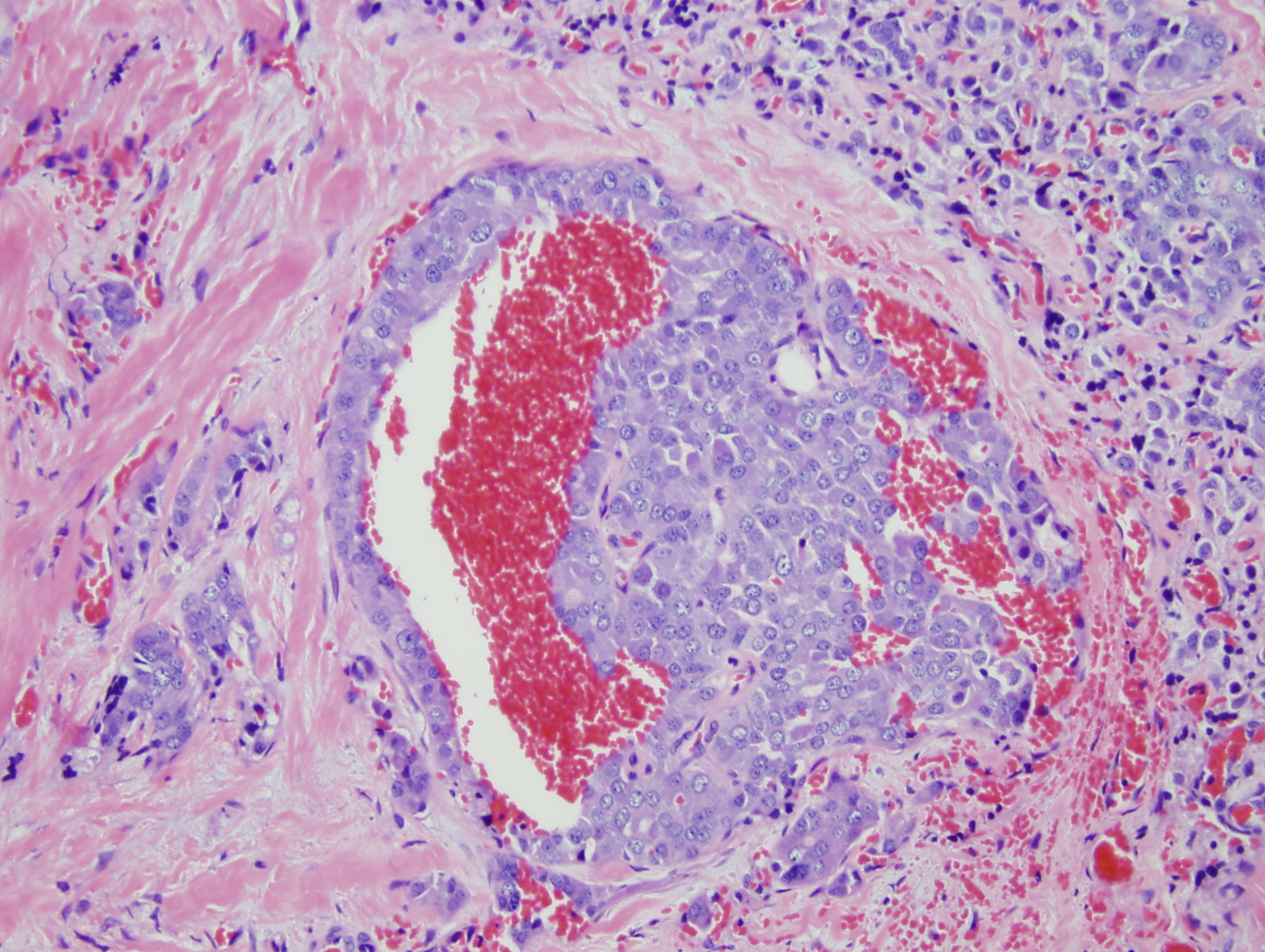
Histologic sections demonstrate nests and cords of tumor cells within a background of hyalinized collagen and amyloid (Figures 1 and 2). The majority of the tumor is composed of polygonal to spindle shaped cells with varying amounts of granular amphophilic cytoplasm, medium-sized, atypical, pleomorphic round-to-oblong nuclei with irregular nuclear membranes, finely granular chromatin and inconspicuous nucleoli (Figure 3). Areas of lymphovascular invasion are also noted (Figure 4).
|
Figure 1: Low power view demonstating nests and cords of tumor cells within a background of hyalinized collagen and amyloid |
Figure 2: Higher power view of tumor admixed with amyloid |
|
Figure 3: High power view of tumor demonstrating polygonal to spindle shaped cells with granular amphophilic cytoplasm, peopmorphic nuclei, fine chromatin and inconspicuous nucleoli, within a background of amyloid |
Figure 4: Lymphovascular invasion
|
Discussion:
Medullary carcinomas of the thyroid are neuroendocrine tumors of C cells, the calcitonin secreting cells of the thyroid. These tumors represent 5-10% of thyroid carcinomas and two main types are seen, sporadic and familial (1). The familial type is associated with multiple endocrine neoplasia (MEN) syndrome types 2A and 2B, as well as familial medullary thyroid carcinoma syndrome (FMTCS). The familial type is seen in 25% of cases (1, 2). This type tends to occur in younger adults, with most pediatric cases occurring due to FMTCS. Usually the tumors are bilateral and multicentric in these cases and they are associated with C-cell hyperplasia. They are typically clinically occult and are discovered after a screening test for serum calcitonin or after RET oncogene mutational analysis. The RET proto-oncogene is located on chromosome 10q11.2 and encodes a tyrosine kinase receptor for glial-derived neurotropic growth factors (3). Mutations at codon 918 are seen in greater than 95% of MEN2B cases (4). The RET mutations are found in all familial forms of MTC and display an autosomal dominant inheritance. Genetic testing of all MTC patients is required, as well as testing of first degree relatives if the patient tests positive. The sporadic type is more prevalent and occurs in middle-aged to older adults. In these cases, there is typically a solitary lesion that is clinically apparent. It is often associated with a paraneoplastic syndrome, such as diarrhea from increased Vasoactive Intestinal Peptide (4).
Laboratory studies that are helpful in the initial diagnosis include serum calcitonin and chromogranin A levels. Calcitonin levels are also helpful in monitoring for recurrence. Only rarely are patients negative for serum calcitonin (5).
Grossly, the tumors can be single or multiple, are typically non-encapsulated, have a firm, gray-tan to yellow appearance, may demonstrate infiltration and may contain hemorrhage or necrosis, particularly in larger lesions. They are most commonly located in the mid-upper portion of the gland due to a higher concentration of C cells in this area.
Microscopically, they may demonstrate a variety of patterns. Classically, they demonstrate a solid proliferation of round, polygonal or spindle shaped cells with granular amphophilic cytoplasm and medium-sized nuclei with punctuate chromatin. The cells are typically arranged in cords and nests and often demonstrate lymphovascular invasion. C-cell hyperplasia is also noted in familial cases. The surrounding stroma is typically highly vascular and contains hyalinized collagen and amyloid with occasional calcifications. Other growth patterns can be seen, including carcinoid-like, paraganglioma-like, trabecular, glandular, pseudopapillary, papillary, inflammatory, spindle cell, oncocytic, small cell, pigmented, mucinous and clear cell variants.
When the tumor metastasizes, the metastases typically resemble the primary tumor microscopically and immunohistochemically and the metastases usually contain amyloid as well. The most common sites of lymph node metastases are the cervical and mediastinal nodes. Distant organs can be involved as well, particularly the lung, liver and skeletal system.
Immunohistochemically, the tumor cells are most commonly positive for keratin, TTF-1, CEA, chromogranin, synaptophysin and calcitonin. They typically do not stain with thyroglobulin. Ultrastructurally, the cells demonstrate multiple dense-core secretory granules in the cytoplasm. Additionally, amyloid filaments and collagen fibers will be seen.
The differential diagnosis of these tumors is rather broad due to the morphologic variety which can be seen. One tumor in the differential is insular carcinoma, also known as poorly differentiated carcinoma. The nesting pattern in these tumors is similar, however insular carcinoma is typically thyroglobulin positive, calcitonin negative and does not demonstrate amyloid. Hurthle cell carcinoma may also be considered in the differential. This tumor resembles the oncocytic variant of MTC, however it is thyroglobulin positive and microscopically, the oncocytic cells are brightly eosinophilic, rather than amphophilic and it lacks the fibrous bands that are typically seen in oncocytic MTC. Another consideration is a hyalinizing trabecular neoplasm which displays a prominent trabecular pattern and hyalinization. These tumors are usually benign but are considered malignant when capsular or vascular invasion is identified. It is important to differentiate these tumors from an encapsulated medullary carcinoma and staining is helpful as these tumors usually stain with thyroglobulin. A mixed medullary-follicular carcinoma could be considered, however this is a somewhat controversial entity. These tumors display morphologic features of medullary carcinoma, including calcitonin positivity, coexisting with features of a follicular neoplasm, including thyroglobulin positivity. Most cases that are considered turn out to be medullary carcinomas with entrapped follicles and thus, secondary incorporation of thyroglobulin by carcinoma cells or medullary carcinomas with a glandular pattern of growth (6). Another consideration would be small cell carcinoma of the thyroid. This entity is morphologically identical to the lung tumor of the same name, however if it is calcitonin positive, it may be regarded as a small cell variant of medullary carcinoma. Additionally, thyroid paragangliomas should be considered. These can have a well-formed "Zellballen" appearance and can occur adjacent to or within the thyroid. These will stain for chromogranin A, synaptophysin, S100 (in sustentacular cells) and neuron specific enolase. They will not stain for calcitonin, thyroglobulin, TTF-1, keratin or Congo red. A final consideration would be metastatic neuroendocrine tumors of various types. These tumors can metastasize to the thyroid and simulate a medullary carcinoma. They will typically display multiple tumor foci and negativity for both calcitonin and CEA helps to rule out the diagnosis of medullary thyroid carcinoma.
The treatment is primarily surgical and entails a total thyroidectomy with cervical node dissection. There is no established role for chemotherapy and radiation is typically used for palliation and residual local disease.
In terms of prognosis, the five year survival rate is 86% (7). Favorable prognostic factors include young age, female sex, familial forms and microcarcinoma (<1 cm). Poor prognostic factors include older age, male sex, sporadic forms and lesions with high mitotic rates, necrosis, small cell type or poor reactivity with calcitonin, usually due to a poorly differentiated lesion.
References:
- Al-Rawi et al. Medullary thyroid carcinoma—update and present management controversies. Ann R Coll Surg Engl. 2006 Sep; 88(5):433-8.
- Lairmore et al. Medullary carcinoma of the thyroid: Current diagnosis and management. Journal of Surgical Oncology. Aug 2006. 7( 2): 92 - 99
- Marini et al. Multiple endocrine neoplasia type 2. Orphanet J Rare Dis. 2006 Nov 14;1:45.
- Sakorafas et al. The genetic basis of hereditary medullary thyroid cancer: clinical implications for the surgeon, with a particular emphasis on the role of prophylactic thyroidectomy. Endocrine-Related Cancer.15 (4):871-884.
- Sand et al. Serum calcitonin negative medullary thyroid carcinoma. World J Surg Oncol. 2006 Dec 21;4:97.
- Luboshitzky et al. Mixed Follicular-Medullary Thyroid Carcinoma: A Case Report. Diagnositc Cytopathology. Jan 2004: 30 (2): 122-124.
- Cupisti et al. Long-term clinical and biochemical follow-up in medullary thyroid carcinoma: a single institution's experience over 20 years. Ann Surg. 2007 Nov;246(5):815-21.

 Meet our Residency Program Director
Meet our Residency Program Director
