Case of the Month
August 2016 - Presented by Nicholas Coley, Mirna Lechpammer and Mingyi Chen
Answer
C. Immunosuppression-related B cell Lymphoproliferative Disorder of the CNS
The tumor in this case is an Immunosuppression-related B cell Lymphoproliferative Disorder of the CNS. The patient’s prolonged immunosuppression facilitated the development of the entity. B cell clonality was verified by identification of rearrangements in IgH and kappa light chain. CNS lymphomas are lymphomas of the diffuse large B cell (DLBCL) type confined to the CNS (Kluin et al., 2008). CNS lymphomas can affect all ages but those that occur in the immunocompromised show no sexual bias and occur about a decade earlier than those in the immunocompetent (which display a 3:2 female predominance) (WHO 2007). Immunodeficiency-associated B cell lymphoproliferative disorders are a new entity recognized by the 2016 Hematopathology WHO.
Which virus is most strongly associated with the development of CNS lymphomas in the immunocompromised?
Epstein Barr Virus (EBV)
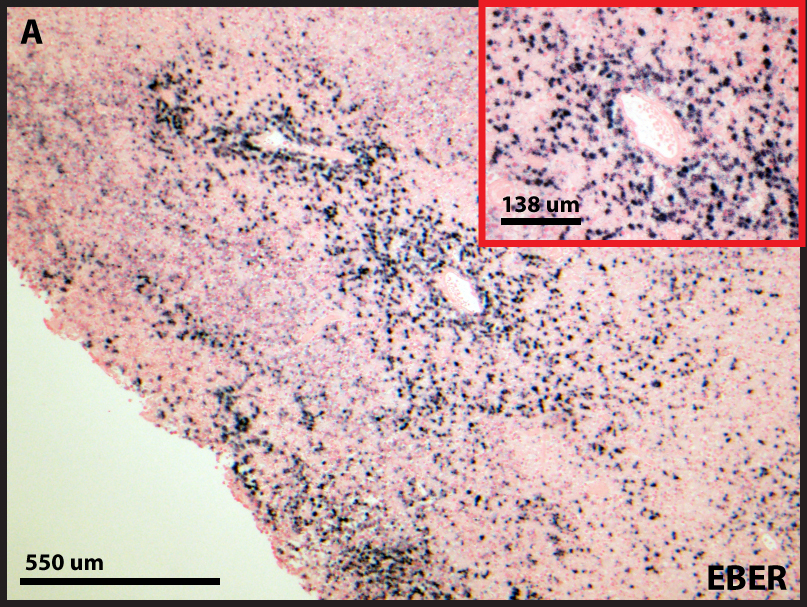
The EBV genome is present in greater than 95% of CNS lymphomas in immunocompromised patients (WHO 2007). In contrast, the EBV genome is present in only 0-20% of cases in the immunocompetent. The lymphoma cells in this case demonstrated EBV infection (Figure 3). In the past, HIV/AIDS patients constituted the majority of immunosuppression-related CNS lymphomas. However, after the advent of triple therapy, patients receiving prolonged pharmaceutical immunosuppression now constitute the largest proportion of immunosuppression-related CNS lymphomas. The molecular mechanisms by which EBV drives the development of lymphoma are beginning to be elucidated. The EBV latent membrane proteins LMP1/LMP2 induce transcription of PD1/PDL1 of the programmed cell death pathway, which promotes a tolerogenic environment by inducing T-cell anergy and evading the surveillance mechanisms of the immune system (Chen et al, 2013).
Click image to enlarge.
Figure 3. The lymphoma cells are infected with the EBV virus as demonstrated by colorimetric in situ hybridization (cISH).
CNS lymphomas are a type of DLBCL that corresponds to a late germinal center maturation stage. The vast majority of CNS lymphomas express follicular markers such as BCL6 and CD10. 60-80% of CNS lymphomas express BCL6 (Montesinos-Rongen et al., 2008). A similar proportion of CNS lymphomas also express the B cell terminal differentiation transcription factor MUM1/IRF4. CNS lymphomas never express the plasma cell markers CD38 and CD138. The cell of origin of CNS lymphomas, however, has not yet been identified. Thus, it is unknown if the entity evolves from intracerebral lymphocytes or lymphocytes that subsequently migrate to the CNS from a secondary site.
Sources Cited
- Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA, Rodig SJ. "PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies". Clin Cancer Res. 2013 Jul 1;19(13):3462-73.
- David N. Louis, Hiroko Ohgaki, Otmar D. Wiestler, Webster K. Cavenee, Peter C. Burger, Anne Jouvet, Bernd W. Scheithauer, and Paul Kleihues. "The 2007 WHO Classification of Tumours of the Central Nervous System". Acta Neuropathol. 2007 Aug; 114(2): 97–109.
- Kluin P, Deckert M, Ferry JA (2008) "Primary diffuse large B-cell lymphoma of the CNS". In: Swerdlow SH, Campo E, Harris NL et al (eds) WHO classification of tumours of haematopoietic and lymphoid tissues. IARC, Lyon, pp 240–241.
- Montesinos-Rongen M, Brunn A, Bentink S et al (2008) "Gene expression profiling suggests primary central nervous system lymphomas to be derived from a late germinal center B cell". Leukemia 22:400–405.

 Meet our Residency Program Director
Meet our Residency Program Director
