Residency Program - Case of the Month
February 2017 - Presented by Ruijun Su and Jeffrey Gregg
Clinical History
A 52-year-old man presented with progressive eosinophilia for two years. His initial eosinophilia was mild, with absolute eosinophils of 2,400/mm3, but the absolute eosinophil count increased to 13,300/mm3 two years later. This was associated with mild normocytic anemia, normal platelet count and no neutropenia or monocytosis. The patient complained of fatigue and muscle pain. Physical examination and imaging studies showed no organomegaly or lymphadenopathy. An extensive workup for infectious diseases was negative for trichinosis, strongyloides, toxoplasma, HIV, schistosomiasis and parasitic infection. Bone marrow biopsy, cytogenetic and molecular studies were performed. Next-generation sequencing (NGS) has also been submitted to further clarify the diagnosis. Pending the NGS data, the patient was treated for 3 months with imatinib, but developed progressive anemia without improvement in the leukocytosis and eosinophilia. After confirming the diagnosis by NGS, therapy was changed to hydroxyurea, which led to an improvement in eosinophils, anemia and clinical symptoms. Allogeneic stem cell transplant is being considered.
Microscopic Description and Immunohistochemistry Studies
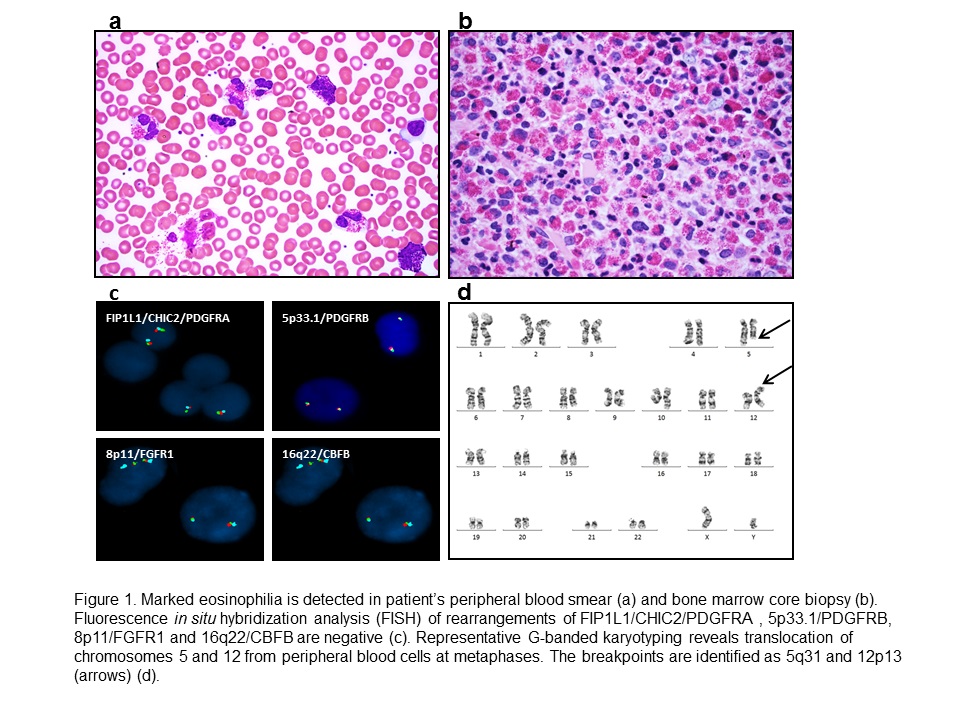
The peripheral blood smear showed leukocytosis with marked eosinophilia, mild basophilia and mild normocytic, normochromic anemia (Figure 1a). Bone marrow biopsy revealed a hypercellular (~90% cellularity) marrow with marked eosinophilia. No increase in immature myeloid precursors, blasts or basophils was detected. There was no overt dysplasia in the remaining trilineage hematopoiesis (Figure 1b). No abnormal mast cells were detected by staining of CD117, CD2, CD25 and tryptase immunostains.
Click on image to enlarge.
Figure 1
Cytogenetic and Molecular Findings
Florescence in situ hybridization (FISH) for MDS markers [-5/del(5q), -7/del(7q), +8 and del(20q)], t(9;22)(q34;q11)/BCR/ABL1, t(15;17)(q24;q21)/PML-RARA, t(8;21)(q22;q22)/RUNX1T1-RUNX1, and inv(16)(p13.3q22)/CBFB were negative. FISH was also negative for rearrangements of FIP1L1/CHIC2/PDGFRA, 5p33.1/PDGFRB, 8p11/FGFR1 and 16q22/CBFB (Figure 1c). No c-KIT (D816V) mutation or T cell receptor gamma gene rearrangement was detected. Metaphase cytogenetic analysis on the bone marrow aspirate detected a t(5;12)(q31;p13) translocation in 9/20 (45%) metaphases (Figure 1d). ETV6-ACSL6 gene fusion was further confirmed by FoundationOne™ Heme assay (Foundation Medicine, Cambridge MA), a next-generation sequencing (NGS) based assay. The current assay utilizes DNA sequencing to interrogate 405 genes as well as selected introns of 31 genes involved in rearrangements, in addition to RNA sequencing of 265 genes. Targeted RNA-seq found 246 reads supporting the fusion event: fusion:5'-ETV6(ex1-1 NM_001987)-ACSL6(ex2-21 NM_015256). No other fusion transcripts were identified.
What is the diagnosis?
Choose one answer and submit.
C. Chronic eosinophilic leukemis, NOS with t(5;12) (q31;p13)/ETV6-ACSL6 genefusion: a novel variant of myeloid neoplasm with eosinophilia
> Learn more about this diagnosis.

 Meet our Residency Program Director
Meet our Residency Program Director
