Resident Program - Case of the Month
November 2018 - Presented by Andrew D. Jones (Mentored by Kristin Olson)
Discussion
Sevelamer is a non-absorbable anion-exchange hydrogel polymer approved by the FDA in 1998 designed to bind phosphate within the GI lumen into an insoluble compound that is passed in the stool.1 It is composed of ammonia fixed on a carbon backbone and associated with an anion in the solid form (e.g. hydrochloride).2
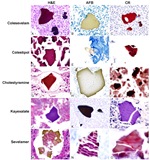
Previous studies have shown that the crystals of sevelamer are morphologically distinct from other ion-exchange resins (e.g. kayexalate, cholestyramine, see Figure 5). They can be distinguished histologically by the characteristic "fish scale" cracked appearance with curved intersecting lines. On H&E, they show two-toned coloration with outlines highlighted in pink and the interior of the crystals orange-yellow.2 Notably, reports have suggested that crystals embedded within areas of mucosal ulceration or ischemia, or within necrotic debris, can show a deep “rusty” brown color (shown in Figure 4).2
Click on image to enlarge.
Figure 5: A comparison between the crystals of various ion exchange resins, seen with hematoxylin and eosin staining (H&E), acid fast bacillus staining (AFB), and Congo red staining (CR). These findings, including the image, were published in Arnold et al.1
Kayexalate (sodium polystyrene sulfonate) crystals, by contrast, have a more rectangular shape with perpendicular intersecting lines and show a characteristic violet color.1,2
Cholestyramine crystals lack internal lines, have a solid single-tone color (typically eosinophilic), and a smooth texture.1,2 Studies have suggested that cholestyramine crystals lack the ability to cause mucosal injury, though they may be associated with areas of injury in patients taking multiple resins.2 Crystals seen in this case are morphologically consistent with sevelamer; the patient was not taking any other ion-binding resins.
Previous reports have implicated sevelamer use as a risk factor for mucosal-associated GI tract injuries. The risk is thought to increase with GI dysmotility and with increasing doses of sevelamer.2 Prior reports have included both upper and lower GI injuries ranging from esophageal ulcerations2,3; chronic gastritis3; duodenal mucosal ulcerations4; inflammatory pseudopolyps of the ileocecal valve3; ascending, transverse, and descending colonic mucosal ulcerations5,6; sigmoid colonic strictures7; colonic mass-like inflammatory lesions8; hematochezia9; and rectal stercoral ulcers,10lesions which span almost the entirety of the GI tract. Limited data have suggested that up to 25% of patients have lesions involving more than one area of the GI tract at presentation.3 To our knowledge, this is the first reported case of acute appendicitis tied to sevelamer use.
There is inadequate data to definitively determine sevelamer to be the cause of the appendicitis in this case; however, the rich abundance of crystals within the appendiceal lumen leaves no doubt that sevelamer was a contributing factor. No other cause of the patient's appendicitis was identified.
Conclusion
Our patient eventually recovered after a nearly month-long hospitalization. She was transitioned back to peritoneal dialysis and subsequently discharged. She remains on sevelamer to prevent hyperphosphatemia and has not presented with further sequelae.
These findings were first published at the American Society for Clinical Pathology Annual Meeting in October, 2018.
References
- Arnold MS, B; Crowder, C. "Colesevelam and Colestipol: Novel Medication Resins in the Gastrointestinal Tract". Am J Surg Pathol. 2014;38:1530-1537.
- Swanson B, Limketkai B, Liu T, Montgomery E, Nazari K. "Sevelamer Crystals in the Gastrointestinal Tract (GIT): A New Entity Associated with Mucosal Injury". Am J Surg Pathol. 2013;37(1686-1693).
- Yuste C, Merida E, Hernandez E, et al. "Gastrointestinal complications induced by sevelamer crystals". Clin Kidney J. 2017;10(4):539-544.
- Magee J, Robles M, Dunaway P. "Sevelamer-Induced Gastrointestinal Injury Presenting as Gastroenteritis". Case Rep Gastroenterol. 2018;12(1):41-45.
- Desai M, Reiprich A, Khov N, Yang Z, Mathew A, Levenick J. "Crystal-Associated Colitis with Ulceration Leading to Hematochezia and Abdominal Pain". Case Rep Gastroenterol. 2016;10(2):332-337.
- Nambiar S, Pillai UK, Devasahayam J, Oliver T, Karippot A. "Colonic Mucosal Ulceration and Gastrointestinal Bleeding Associated with Sevelamer Crystal Deposition in a Patient with End Stage Renal Disease". Case Rep Nephrol. 2018;2018:4708068.
- Kim J, Olson K, Butani L. "Sevelamer crystals in the mucosa of the gastrointestinal tract in a teenager with end-stage renal disease". Pediatr Nephrol. 2016;31(2):339-341.
- Bansal V, Aggarwal P, Mittal A, Vachhani M, Aggarwal P, Aggarwal N. "Colonic Mass Secondary to Sevelamer-Associated Mucosal Injury". ACG Case Rep J. 2017;4:e92.
- Chintamaneni P. "Hematochezia Associated with Sevalamer-Induced Mucosal Injury". ACG Case Reports Journal. 2014;1(3).
- Madan P, Bhayana S, Chandra P, Hughes J. "Lower gastrointestinal bleeding: Association with Sevelamer use". World J Gastroenterol. 2008;14(16):2615-2616.

 Meet our Residency Program Director
Meet our Residency Program Director
