Resident Program - Case of the Month
January 2020 - Presented by Guofeng Gao (Mentored by Grace F. Monis)
The case is a classic case of recurrent FSGS with histologically characteristic finding in renal biopsies (focal areas of sclerosis of some glomeruli adjacent to other intact glomeruli). Most FSGS cases (approximately 80%) are idiopathic (or primary), while the other 20% are due to known causes (secondary): genetic mutations in specific podocyte genes, drug and hemodynamic adaptive response. It is hypothesized that the idiopathic FSGS is caused by a circulating plasma “permeability factor” of unknown origin which leads to injures of the filtration barrier and/or increased glomerular permeability; however, this circulating plasma “permeability factor” is present in some but not all patients with FSGS. Some studies showed this circulating pathogenic plasma “permeability factor” to be the soluble urokinase plasminogen activator receptor (suPAR, a glycoprotein of molecular weight of 30–50 kDa) with elevated levels in patients with idiopathic FSGS. This hypothesis is supported by studies demonstrating that FSGS may recur in a renal allograft and the main source of circulating plasma “permeability factor” (suPAR) is bone marrow-derived immature myeloid cells.
FSGS has several histological variants: cellular, collapsing, tip lesion, perihilar, and not otherwise specified. Although these variants appear to have different clinical presentations and treatment response, podocyte injury and depletion are the central mediators for all FSGS. End Stage Renal Disease is expected in most patients with FSGS within 3 - 7 years, leaving transplantation the desirable option to avoid lifelong dialysis and for better life quality. However, to make things worse, unfortunately, the majority of transplanted patients will experience recurrence of FSGS in the renal allograft and worsening renal functions. If untreated, FSGS will ultimately lead to permanent graft loss within months. Patients with kidney transplant due to idiopathic FSGS have the highest risk of post-transplant recurrence of FSGS, because such patient still have the pathogenic “permeability factor” circulating in their plasma. Those who lose grafts due to recurrent FSGS have >80% chance of developing recurrent FSGS again in subsequent kidney transplants. Therefore, Therapeutic Plasma Exchange (TPE) is used to remove this circulating pathogenic plasma “permeability factor” to control recurrent FSGS to prevent or delay kidney transplant loss.
Besides original or recurrent FSGS, there are many risk factors for recurrent FSGS: younger age (6 - 15 years), short duration of native kidney disease (<3 years), pre-transplantation heavy proteinuria, bilateral native nephrectomy, nonblack race, and kidney from a living donor. The patient of the above case has several of such risk factors: her presentation with nephrotic syndrome and native kidney biopsy showing FSGS at a younger age of 14, nephrotic range proteinuria, Caucasian female, and kidney from a living donor (her cousin), which put her at significantly increased risk for developing recurrent FSGS in the transplant kidney. Recurrent FSGS can happen as early as a few hours post-transplant and as late as two years post-transplant. This patient probably had an early FSGS recurrence, as reflected by the deteriorating kidney function a month after transplantation and transplant kidney biopsy showing diffuse foot process effacement, possible early podocyte injury caused by the circulating plasma “permeability factor” leading to injures of the filtration barrier and/or increased glomerular permeability, ultimately FSGS.
Recurrent FSGS in the transplanted kidney is diagnosed histologically on transplant kidney biopsy or clinically when nephrotic range proteinuria develops in the post-transplant patients with a history of FSGS in the native kidney or in a previous allograft. FSGS can also be suspected when patients with a history of FSGS have less severe but persistent proteinuria (> 0.5 g/day) within the first 10 days post-transplant. This patient had immediate post-transplant graft function with baseline sCr 1.3 -1.5 mg/dL, but a month later she had early recurrent FSGS reflected by rise of Urine Albumin-to-Creatinine Ratio to 4 gm/gm Cr, which was promptly treated by daily TPE followed by weaning and oral prednisone. However, proteinuria worsened after TPE was tapered down to once a week, so she had developed clinically apparent FSGS dependent on TPE. Although with TPE, her kidney worsening still progressed to biopsy-proved recurrent FSGS on repeat transplant kidney a year later. Now she undergoes regular TPE every 2 weeks.
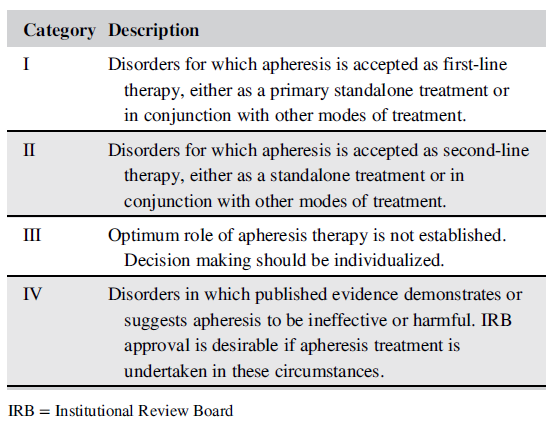
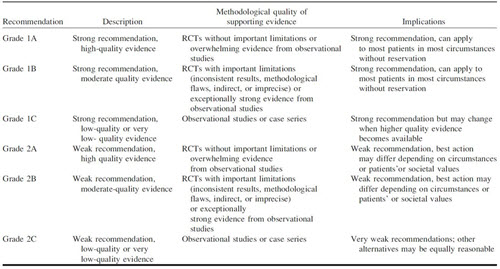
TPE does not benefit patients with primary FSGS in native kidneys with proteinuria >3g/day, for which corticosteroids remain the mainstay of treatment. However, recurrent FSGS often responds to TPE, in conjunction with high dose corticosteroids, IVIG, cyclosporine A or other immunosuppression like tacrolimus, rituximab, and mycophenolate mofetil. The American Society for Apheresis (ASFA) has published practice guidelines on the use of therapeutic apheresis in the Journal of Clinical Apheresis Special Issue. There are four categories of indications for therapeutic apheresis (Table I). Per the 2019 ASFA guidelines, its use to treat FSGS is ASFA Category I and this means TPE is a first-line therapy, either as a primary standalone treatment or in conjunction with other modes of treatment) to treat FSGS. ASFA guidelines also publish Grading Recommendations for Therapeutic Apheresis (Table II) based on systematic review of the literature and the quality of published evidence. Per the 2019 ASFA guidelines, the use of TPE to treat FSGS is ASFA Grading Recommendations: 1B (Strong recommendation, moderate-quality evidence).
The underlying causes of secondary FSGS should be treated whenever possible. The main goal of recurrent FSGS treatment is to achieve a complete or partial remission of proteinuria and prevent/delay premature allograft loss, because TPE can remove the still circulating pathogenic “permeability factor” from patients’ plasma or decreasing the plasma concentration of this “permeability factor”.
Table I: Indications for Therapeutic Apheresis (from Schwartz et al. 2019)
Although it has been showed that pretransplant TPE appears to prevent or delay recurrence in high-risk patients, more commonly TPE is started as soon as recurrent FSGS is diagnosed in order to halt or delay the process and maintain kidney function. Vascular access may be obtained through arteriovenous fistulas, grafts used for dialysis or ports surgically placed in patients. Volume to be treated every time is 1-1.5 total plasma volume, with both albumin, plasma or more commonly a combination of the two as replacement fluid. The frequency of TPE treatment is quite variable and should be decided on a case by case basis and is guided the patient’s overall status. It can be done by 3 daily TPEs to begin with, followed by at least six more TPEs in the subsequent 2 weeks, for a minimum of nine procedures. Or it can be done using with the following intense/maintenance approach: three per week for the first 3 weeks, followed by 2 TPEs per week for 3 weeks, one TPE per week until month 3, 2 TPEs per month until month 5, and once per month until month 9, but with concomitant immunosuppression treatment.
Table II: Grading Recommendations (from Schwartz et al. 2019)
(Click table to enlarge.)
REFERENCES
Alfano M, Cinque P, Giusti G, Proietti S, Nebuloni M, Danese S, D'Alessio S, Genua M, Portale F, Lo Porto M, Singhal PC, Rastaldi MP, Saleem MA, Mavilio D, Mikulak J. "Full-length soluble urokinase plasminogen activator receptor down-modulates nephrin expression in podocytes". Sci Rep. 2015 Sep 18;5:13647.
Gohh RY, Yango AF, Morrissey PE, Monaco AP, Gautam A, Sharma M, McCarthy ET, Savin VJ. "Preemptive plasmapheresis and recurrence of FSGS in high-risk renal transplant recipients". Am J Transplant 2005; 5: 2907–2912.
Hahm E, Wei C, Fernandez I, Li J, Tardi NJ, Tracy M, Wadhwani S, Cao Y, Peev V, Zloza A, Lusciks J, Hayek SS, O'Connor C, Bitzer M, Gupta V, Sever S, Sykes DB, Scadden DT, Reiser J. "Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease". Nat Med. 2017 Jan;23(1):100-106.
Huang J, Liu G, Zhang YM, Cui Z, Wang F, Liu XJ, Chu R, Zhao MH. "Urinary soluble urokinase receptor levels are elevated and pathogenic in patients with primary focal segmental glomerulosclerosis". BMC Med. 2014 May 20;12:81.
Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, Pham HP, Schneiderman J, Witt V, Wu Y, Zantek ND, Dunbar NM, Schwartz GEJ. "Guidelines on the use of therapeutic apheresis in clinical practice –evidence-based approach from the Writing Committee of the American Society for Apheresis: the eighth special issue". J Clin Apher 2019; 34:171–354.
Sharma M, Sharma R, McCarthy ET, Savin VJ. "'The FSGS factor:' enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma". J Am Soc Nephrol 1999; 10: 552–561.

 Meet our Residency Program Director
Meet our Residency Program Director
