Resident Program - Case of the Month
Diagnosis:
Post-transplant lymphoproliferative disorder (PTLD) arises in recipients of solid organ or stem cell allografts as a consequence of immunosuppressive therapy and is commonly driven by Epstein-Barr virus (EBV) infection. PTLD frequently occurs less than 1 year after transplantation, especially in EBV-seronegative recipients who acquire a post-transplantation primary infection. However, studies have shown that the median time to the development of PTLD is several years and may occur more than 10 years after transplantation. It has also been reported that EBV-negative PTLD tends to present later.
Based on the histologic features of the lesion, PTLD is categorized as non-destructive PTLD, polymorphic PTLD, monomorphic PTLD, and classic Hodgkin lymphoma (CHL) PTLD.
Non-destructive PTLD commonly involves the tonsils and lymph nodes and, as indicated in its name, shows preservation of the overall architecture of the organ that is involved. Non-destructive PTLD is further divided into three histologic subtypes: infectious mononucleosis, plasmacytic hyperplasia, and follicular hyperplasia. In general, lesions consist of a mixed lymphocytic and plasmacytic infiltrate showing a spectrum of B-cell maturation.
Polymorphic PTLD (answer choice D) commonly involves lymph nodes and extranodal sites including the gastrointestinal tract. Lesions consist of a mixed lymphocytic and plasmacytic infiltrate but are by definition associated with effacement of the organ architecture. While a spectrum of B-cell maturation is present, atypical immunoblasts may be present in polymorphic PTLD. However, sheets of large atypical cells, as seen in the current case, should be absent.
Monomorphic PTLD, which accounts for most cases of PTLD, results from the proliferation of a monoclonal cell type and occurs in a variety of body sites. Monomorphic PTLD is subclassified as the B-cell or T/NK-cell neoplasm recognized in immunocompetent hosts which it fulfils conventional criteria for. The majority of cases of monomorphic PTLD are B-cell neoplasms, most commonly diffuse large B-cell lymphoma (DLBCL), and are EBV-positive. Monomorphic T/NK-cell PTLDs are typically EBV-negative.
CHL PTLD is often of the mixed cellularity subtype of CHL, demonstrates the typical CHL immunophenotype, and is EBV-positive in the vast majority of cases.
The diagnosis of PTLD, regardless of type, depends on a patient’s history of organ transplantation. Furthermore, the presence of EBV-positive cells may help distinguish it from other competing diagnoses. If present, detection can be done by EBV-encoded RNA by in situ hybridization (EBER-ISH).
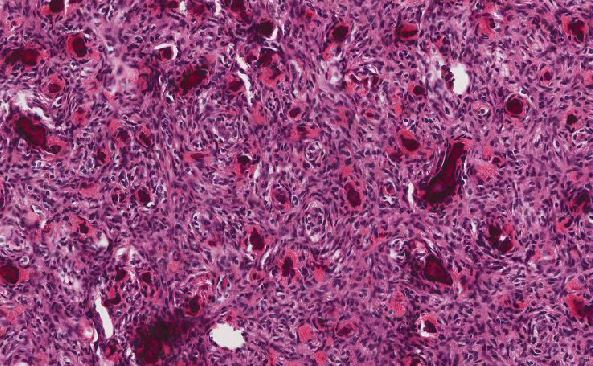
As presented in this case, the patient’s history of organ transplantation in addition to the morphology and immunophenotype of the omental tumor was most consistent with a diagnosis of monomorphic PTLD, DLBCL type, despite being EBV-negative by EBER-ISH. In the initial differential diagnosis of neoplasms with high-grade morphology, the possibility of carcinoma and sarcoma (answer choice B) should be considered. An epithelial malignancy that commonly presents with abdominoperitoneal metastasis and affects older women is high-grade serous carcinoma of tubo-ovarian origin (answer choice A). However, given the immunohistochemical (IHC) staining pattern of the neoplasm in this case, particularly the lack of expression with cytokeratin cocktail AE1/AE3, carcinoma may be ruled out. Similarly, sarcoma is unlikely as the immunophenotype strongly supports lymphoma. If this were not the case, additional IHC studies would be warranted to investigate additional entities, including a possible high-grade sarcoma.
The IHC workup in this case also aided in further classification and prognostication of the patient’s DLBCL. According to the Hans algorithm which utilizes CD10, BCL-6, and MUM1 IHC stains for cell of origin assignment, criteria were met for a non-germinal center B-cell (GCB) type of DLBCL. Based on the expression of BCL-2 and C-MYC by IHC in a majority of neoplastic cells, this DLBCL also represented a double expressor lymphoma. Non-GCB and double expressor lymphomas have been associated with worse prognosis.
Of note, fluorescent in situ hybridization analysis of MYC, BCL2, and BCL6 genes was subsequently performed on tissue from the core needle biopsy. BCL6 gene rearrangement was detected while no significant BCL2 or MYC gene rearrangements were observed. This indicated no evidence of a double hit lymphoma (having MYC and BCL2 or BCL6 rearrangements) or triple hit lymphoma (having MYC, BCL2, and BCL6 rearrangements), which are associated with poorer clinical outcomes.
References:
- Campo E, Harris NL, Pileri SA et al. "WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues". IARC Who Classification of Tum; 2017.
- Medeiros LJ, Lin P, Miranda RN. "Posttransplant Lymphoproliferative Disorder, Early Lesions and Polymorphic". ExpertPath. Updated May 26, 2019.
- Medeiros LJ, Lin P, Miranda RN. "Posttransplant Lymphoproliferative Disorder", Monomorphic. Updated May 25, 2019.
- Hans CP, Weisenburger DD, Greiner TC, et al. "Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray". Blood. 2004;103(1):275-82.
- Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452-9.
- Horn H, Ziepert M, Becher C, et al. "MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma". Blood. 2013;121(12):2253-63.
- Kumar V, Abbas AK, Aster JC. "Robbins and Cotran Pathologic Basis of Disease". W.B. Saunders Company; 2014.
- Amin MB. "AJCC Cancer Staging Manual, Seventh Edition". American College of Surgeons; 2010.

 Meet our Residency Program Director
Meet our Residency Program Director