Resident Program - Case of the Month
February 2021 – Presented by Dr. Ryan Thomas (Mentored by Dr. Zarir Karanjawala)
Case Discussion:
Blastic plasmacytoid dendritic cell neoplasm (also known as agranular CD4+/CD56+ hematodermic neoplasm) is an aggressive disease involving the skin and bone marrow, often with leukemic dissemination and lymph node involvement1,2. The neoplastic cells are derived from the precursors of plasmacytoid dendritic cells, which normally produce Type I interferons and serve immune functions such as antiviral immunity, antitumor immunity, and peripheral tolerance3. The disease is rare and effects mainly elderly adults with a male predilection of about 3-4:1, though it can occur in any age range, including childhood1,2.
Patients commonly present with solitary or multiple bruise-like skin lesions or skin nodules. Bone marrow involvement will occur as the disease progresses. Other findings include regional lymphadenopathy, peripheral blood involvement, and cytopenias.
The histologic findings of the bone marrow in blastic plasmacytoid dendritic cell neoplasm include a diffuse infiltrate of medium-sized immature/blastic cells with irregular nuclei containing one to several nucleoli1,2. The cytoplasm is scant and agranular, may contain perinuclear vacuoles, and may have pseudopod-like projections (see figure 3 and figure 4). Histologic examination of skin lesions will show tumor cells predominantly in the dermis with sparing of the epidermis and possibly extending into the subcutaneous fat.
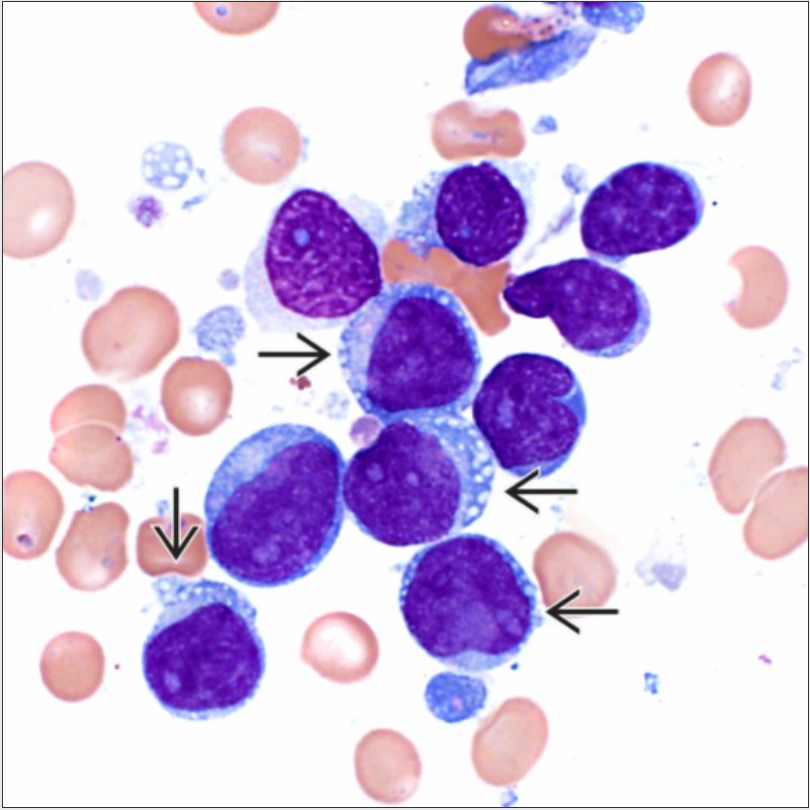
Figure 3: The cytoplasm in the neoplastic cells may contain perinuclear vacuoles (arrows). Image courtesy of ExpertPath
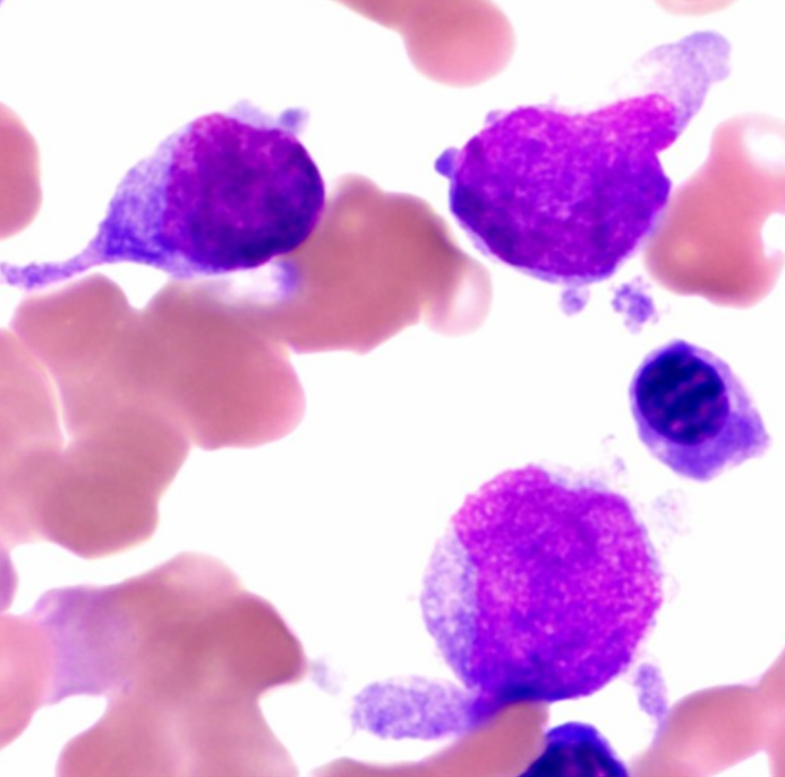
Figure 4: The cytoplasm of the neoplastic cells can also exhibit pseudopod formation as shown. Image courtesy of ExpertPath
The neoplastic cells express CD4, CD56, CD123, TCL1, and BDCA2 (CD303) by immunohistochemistry. They are negative for CD34, myeloperoxidase, lysozyme, and B- and T-cell lineage-specific markers1,2,3,4. There is no specific chromosomal aberration associated with this neoplasm, though the neoplastic cells will often have complex karyotypes.
Diagnosis of blastic plasmacytoid dendritic cell neoplasm presents a challenge due to the various morphologies (including pseudomonoblastic and pseudolymphoid appearances) of the neoplastic cells as well as possible expression of markers seen in other cell lineages (such as CD33, CD7, CD22, CD79a, etc.)4. The immunophenotype seen can be similar to non-plasmacytoid dendritic cell leukemia, especially AML or immature ALL. It is therefore important to keep this entity in mind and have a high index of suspicion when faced with an immature neoplastic population expressing non-specific lineage markers along with HLA-DR, CD4, and CD-56, with confirmation of the diagnosis using more specific markers like TCL-1, or BDCA2.
Additionally, about 10–20% of BPDCN patients are also affected by other myeloid neoplasms, including myelodysplastic syndromes and chronic myelomonocytic leukemia (CMML)5,6,7. It is important to note that there can be an expansion of mature plasmacytoid dendritic cells (which are negative for CD56) in these related entities as well, particularly in CMML5,6.
Blastic plasmacytoid dendritic cell neoplasm runs an aggressive course, with relapse being common after initial response to chemotherapy as well as development of a fulminant leukemic phase. Median survival is 12-14 months.
References:
- Swerdlow, S., Campo, E., Harris, N., Jaffe, E., Pileri, S., Stein, H. and Thiele, J., 2017. WHO Classification Of Tumours Of Haematopoietic And Lymphoid Tissues. 4th ed. Lyon: International Agency for Research on Cancer, pp.145-147.
- Reichard, K., 2021. Blastic Plasmacytoid Dendritic Cell Neoplasm. [online] App.expertpath.com. [Accessed 25 January 2021].
- Jegalian, A., Buxbaum, N., Facchetti, F., Raffeld, M., Pittaluga, S., Wayne, A. and Jaffe, E., 2010. Blastic plasmacytoid dendritic cell neoplasm in children: diagnostic features and clinical implications. Haematologica, 95(11), pp.1873-1879.
- Garnache-Ottou, F. et al, 2019. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients?. Blood Advances, 3(24), pp.4238-4251.
- Brunetti, L., Di Battista, V., Venanzi, A., Schiavoni, G., Martelli, M., Ascani, S., Mecucci, C., Tiacci, E. and Falini, B., 2017. Blastic plasmacytoid dendritic cell neoplasm and chronic myelomonocytic leukemia: a shared clonal origin. Leukemia, [online] 31(5), pp.1238-1240. [Accessed 2 February 2021].
- Espasa, A., Sorigue, M., Tapia, G., Cabezon, M., Vergara, S., Raya, M., Navarro, J., Junca, J., Zamora, L. and Xicoy, B., 2020. Chronic myelomonocytic leukemia and blastic plasmacytoid dendritic cell neoplasm. A case report and systematic review. Cytometry Part B: Clinical Cytometry, [online] [Accessed 2 February 2021].
- Lls.org. 2019. Facts About Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN). [online] [Accessed 2 February 2021].
- Khoury, J., 2018. Blastic Plasmacytoid Dendritic Cell Neoplasm. Current Hematologic Malignancy Reports, [online] 13(6), pp.477-483. [Accessed 25 January 2021].

 Meet our Residency Program Director
Meet our Residency Program Director
