New procedure transforms grafts used in bladder augmentation surgeries
UC Davis pediatric urologist Eric Kurzrock has devised a new procedure to create graft tissues with functioning blood vessels needed in bladder augmentation surgeries. His innovative method solves a common problem in bioengineered graft implants, the challenge of tissue contraction caused by inadequate blood supply.

Kurzrock is the chief of pediatric urologic surgery and professor of urology and pediatrics at UC Davis Children’s Hospital. His study on vascularized grafts for bladder augmentation was published in the Journal of Tissue Engineering and Regenerative Medicine.
Challenges with current bladder wall substitutes
Patients with spina bifida or spinal cord injury may have a neurogenic bladder caused by dysfunctional nerve messaging between the bladder and the nervous system. This can lead to problems with urination. These patients may need an operation to enlarge their bladder, called “augmentation”.
Current bladder enlargement techniques require an extensive abdominal operation and use sections from the intestine, colon or stomach as a substitute for bladder tissue. Tissue replacements may lead to undesirable mucous production, recurrent urinary tract infection, kidney stone formation and electrolyte imbalance.
The ideal substitute for the bladder wall would be bioengineered tissue.
Bladder augmentation using small, bioengineered grafts has been successful in both small and large animal models. However, results have not been consistent when large grafts were used in large animal models due to graft contraction caused by an inadequate blood supply.
“We recognized that the lack of functioning blood vessels was limiting the progress of using larger grafts,” Kurzrock said.
To overcome this graft contraction issue, Kurzrock and his team devised an implant procedure that takes place in several stages to create bladder tissue with functioning vessels.
Extending blood vessel connections
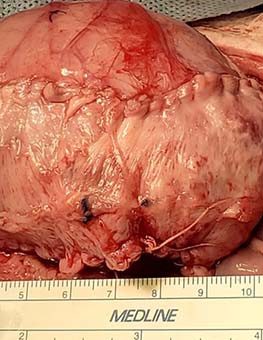
In 2015, Kurzrock placed a small graft with blood vessels into the bladder of a chimeric mouse model. The blood vessels from the host grew connections to the graft’s blood vessels, a process known as “inosculation.”
After success in the mouse model, Kurzrock extended the experiment to a large animal model, specifically pigs. After trying various methods to engineer blood vessels in large grafts, Kurzrock devised a new technique to safely place and vascularize a graft in the body of a pig. The graft is made up of pig tissue, processed to remove the cells while retaining the extracellular structure. This process prevents rejection and an immune response.
“We had to think of a place for the graft to be safely put, get vascularized, and later harvested without destroying other tissues. It turns out the ideal location is on top of the rectus muscle. When we make an incision for bladder surgery, surgeons can easily access the space between the fascia and rectus muscle, so the patients do not have any extra incisions,” Kurzrock said.
The rectus muscle is an abdominal muscle commonly referred to as the six-pack. Growing grafts on top of this muscle is exceptionally uncommon. Surgeons rarely have a reason to separate the rectus muscle from the fascia connective tissue, so it is a potential space not seen by many.
Kurzrock was able to put a graft on top of the rectus muscle, leave it for two months, and then retrieve it. He even discovered that they could do that process multiple times with more grafts in the same animal.
“The fact that we could do this in a pig model that stayed alive after the procedure with very few complications suggests that translating it to humans should be feasible,” Kurzrock explained.
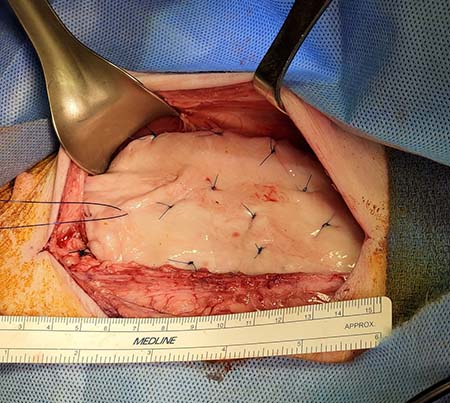
The good news came when the grafts were placed on the bladder and within only two weeks the graft’s vessels were connected with the host’s blood vessels. This inosculation is critical for the success of the grafts when used in bladder enlargement.
The vessel connections provide channels for the blood to flow through the graft within days of the transplant and prevent contraction. Grafts bioengineered with pre‐existing vessels allow these connections to form between the graft and host vessels.
“It was great to see blood flow to the middle of the graft. You have this large thin piece of tissue with lots of small vessels, and you rely on the body to connect the blood vessels at the microscopic level and allow them to grow together. It is vital that this happens fast enough that the graft does not die,” Kurzrock said.
By three months, the bladder grafts had mature, smooth muscle and a structure similar to the native bladder.
The body as graft incubator
Kurzrock suggested that this multi-stage technique may allow multiple grafts to grow over years. This is especially important for pediatric patients as they grow and their bladder increases in size.
“We may grow a graft in the rectus space and then put it on the bladder. Then implant another graft in the rectus space to be matured and a few years later take that graft and add it to the bladder,” Kurzrock said.
Co-authors of the study are Stephanie Osborn, Leanna Mah, Erica Ely, Stefania Ana, Christina Huynh, Naveena Ujagar, Serena Chan, Philip Hsiao, Jonathan Hu, Yvonne Chan, and Blaine Christiansen.
This work was supported by grants from Shriners Hospitals for Children (85,210) and the Department of Defense – Spinal Cord Injury Research Program (W81XWH-18-10,657).
Related stories