UC Davis Health to begin region’s first-of-its kind clinical trial to treat complex aortic aneurysms
Vascular surgeon receives special approval from FDA to use new device in minimally invasive procedures to repair aortas
Steven Maximus, director of aortic surgery at UC Davis Health, has received special approval from the Food and Drug Administration (FDA) to use a new device in clinical trials to treat complex aortic aneurysms.
Maximus is one of 18 vascular surgeons in the United States, and the only one in Northern California, to be granted the physician-sponsored investigational device exemption (IDE) . The exemption is specifically for the use of an endovascular aortic device, which can repair an artery without making an incision.
What is an investigational device exemption?
An IDE is regulatory permission to allow an investigational device to be used in a clinical study to collect safety and effectiveness data. A physician-sponsored IDE is one that allows a device that is not currently approved by the FDA to be used on patients and studied in a clinical trial. A physician-sponsored IDE means the surgeon is both the sponsor and the investigator, as opposed to commercially sponsored IDEs that are requested by device manufacturers.
The clinical trial is significant because the procedure is minimally invasive and can result in life-saving care.
“With this physician-sponsored IDE, we are evaluating the safety and effectiveness of our physician-modified aortic endograft (device) to determine if it should be used in a larger population,” Maximus explained.
How will the clinical trial work?
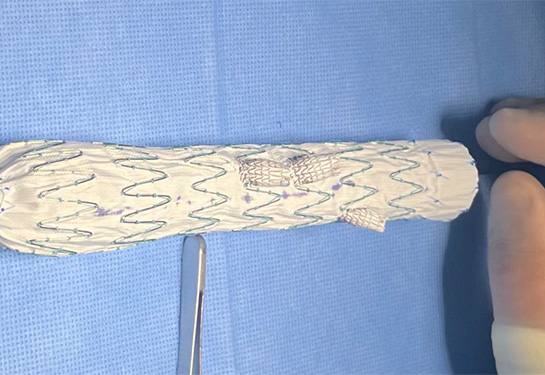
Physician modified aortic endografts (PMEGs) involve the modification of commercially available endografts in the operating room under sterile conditions. Each device is customized by the physician to suit a particular patient's anatomy.
This clinical trial will evaluate the safety and effectiveness of PMEGs in treating three complex aortic aneurysms:
- Thoracoabdominal aneurysms: A type of aortic aneurysm where the wall of the aorta, the largest blood vessel in the body, weakens and bulges. This aneurysm extends into both the chest and abdominal regions of the aorta.
- Paravisceral aneurysms: An aneurysm that is in the aorta running from the aortic hiatus to the renal arteries.
- Pararenal aneurysms: A classification of an aortic aneurysm where there is no normal aortic segment between the renal artery and the proximal border of the aneurysm.
“Treating patients with these aortic aneurysms presents many challenges due to the size, shape and location,” Maximus explained. “Current treatment of these pathologies is limited, and a substantial population of patients are precluded from open surgery due to age, comorbidities, or other concerns. Currently, there is no approved customized endovascular device to treat complex aortic aneurysms in the United States — and off-the-shelf solutions are not customized to patient anatomy, creating significant limitations.”
Treating patients with these aortic aneurysms presents many challenges due to the size, shape and location. Current treatment of these pathologies is limited, and a substantial population of patients are precluded from open surgery due to age, comorbidities, or other concerns.”—Steven Maximus
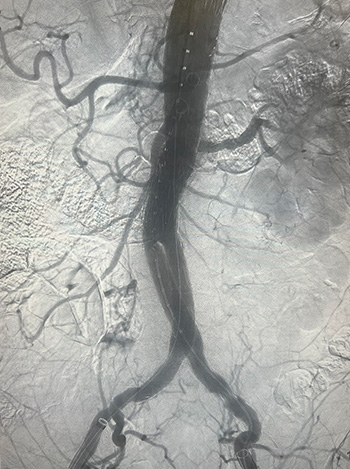
During the trial, Maximus and his team will evaluate their customized PMEGs for the endovascular repair of complex aortic aneurysms. They will also examine the short- and long-term outcomes of the technique.
“We are excited to offer our patients a minimally invasive option for conditions that previously could only be treated with major open surgery,” Maximus said. “With this procedure, we have the potential to create a better experience for our patients and improve their outcomes.”