Case of the Month
September 2016 - Presented by Andrew Jones and Morgan Darrow
Diagnosis
Primary pulmonary lymphoepithelioma-like carcinoma (LELC) is a rare and recently characterized form of lung cancer. A type of non-small cell carcinoma (NSCLC), it was first described in 1987 by Begin et al and over 300 cases have been reported in the English literature since then. LELC represents up to 0.9% of all primary lung carcinomas.
Unlike other NSCLC, LELC has a slight predominance for women under 60 years and is more common in nonsmokers than smokers. LELC is highly associated with the Epstein-Barr virus (EBV) in Asian patients. (This association is weaker in non-Asian patients.)
The typical presentation for LELC is exemplified by the case study above: patients either present with a progressively worsening dry cough with bloody sputum or are asymptomatic (and the tumor is discovered as an “incidentaloma”). Rarely, patients present with more severe symptoms such as weight loss, chest pain, and fever.
On imaging, the lesion is likely to be seen as a solitary peripheral mass, or so-called “coin lesion.” Only rarely do patients present with advanced distant metastatic disease. Grossly, the resected tumors are well-circumscribed and firm with a homogeneous yellow-white appearance. Histologically, the tumors are well-demarcated from the surrounding lung parenchyma and consist of syncytial sheets and nests of large epithelioid cells admixed with abundant lymphocytes (hence the aptly descriptive “lymphoepithelioma” designation).
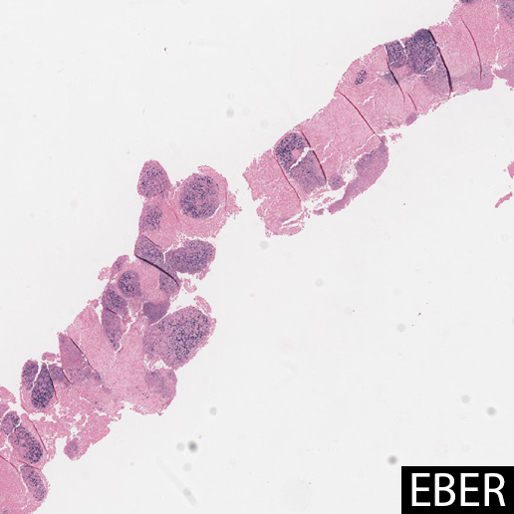
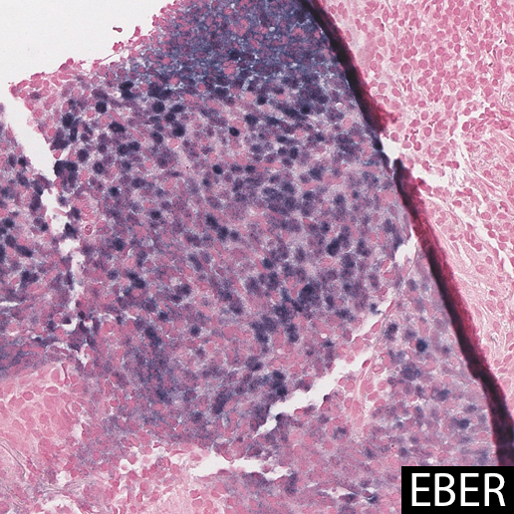
In most cases, and particularly in East Asian patients, in situ hybridization for EBER shows diffuse positivity in the epithelioid cells. The largest case series of LELC in non-Asian Westerners (n=6) showed no association with EBV, suggesting a possible alternate pathogenesis. One case report showed that EBV DNA in peripheral blood was elevated prior to resection, and undetectable after surgery, suggesting a possible serologic surrogate marker for treatment response. Other studies have shown that tumor cells contain a single episomal form of EBV, suggesting EBV infection preceded clonal tumor cell expansion.
EBER staining highlights EBV viral integration in the nuclei of the epithelioid cells, but not the lymphocytes.
Click images to enlarge.
 |
 |
The epithelioid cells are also typically positive for cytokeratin AE1/AE3, cytokeratin 5/6, p63, Napsin A, and Bcl-2, while negative for TTF-1, CK7, CK20, EMA, chromogranin A, synaptophysin, and CD56. This suggests that the epithelioid component of the tumor is likely of squamous origin. Limited studies have shown a relatively low rate of EGFR and EML4-ALK mutations in LELC, especially in East Asian patients, again highlighting LELC’s distinction from other lung cancers. In one case report of a LELC/adenocarcinoma collision tumor, the authors used EGFR, ALK, c-Met, and EBER to show the two elements of the tumor arose from different cell origins. Of note, the tumor infiltrating lymphocyte (TIL) population has been described as predominantly CD8+ cytotoxic T-cells with no EBV-staining.
Several studies suggest that LELC are slow-growing tumors. In one case report, a patient presented with an incidental lung nodule that had no increased FDG uptake on PET. Two years later, the tumor had increased FDG avidity, and was then removed. The usual early stage at presentation similarly seems to suggest an extended period of indolence.
Treatment for early disease universally includes surgical excision; the roles of adjuvant chemotherapy and radiation are less clear, in part due to the paucity of cases and the even rarer situation of advanced disease. For patients with incomplete resection or advanced disease at presentation, recurrence is possible. For those with tumors resectable to clear surgical margins, disease-free survival (DFS) and overall survival (OS) are good: 2- and 5-year survival rates are estimated in one series to be 88% and 62% respectively. Factors like early stage tumors, normal LDH levels, normal serum albumin levels, no lymph node metastasis, and complete resection have all been significantly associated with better OS. A different study found tumor recurrence and tumor necrosis were both markers of poor prognosis in OS, as was EBV serum DNA level of > 10,000 copies / mL. Another study suggested that the pretreatment monocyte-to-lymphocyte ratio (MLR) could be used as a prognostic marker for LELC patients, as it has for other lung cancers. A higher MLR (≥ 0.262) was significantly associated with poor OS and DFS. This same study found that tumor-associated macrophages were significantly correlated with worse survival outcomes (though this data has not yet been published).
Also linked to poor disease-free survival is tumor over-expression of programmed cell death-ligand 1 (PD-L1), a common driver mutation in other NSCLC. While not necessarily a significant prognostic indicator in other NSCLC, PD-L1 overexpression in LELC has been shown to significantly decrease DFS, but not OS. (This same study showed that both nodal and distant metastasis were independent prognostic factors for DFS, but not OS.) Mutations of PD-L1 promote proliferation of cancer cells and induce immune evasion of tumor cells, leading to lymphocyte “exhaustion” and immune tolerance. Confirmed in another study, this suggests that patients with PD-1/PD-L1 mutations may benefit from treatment with targeted therapies.
One case report suggested possible spontaneous regression of a LELC tumor in a patient who began taking an SSRI (similar to what has been suggested with colorectal tumors), however the results have not been verified. Another case report described a patient who presented with nephrotic syndrome, which was attributed to a paraneoplastic effect of a subsequently discovered LELC. Another linked the syndrome of inappropriate antidiuretic hormone (SIADH) to a case of LELC. In all three cases, in situ hybridization for EBV was negative.
The differential diagnosis for the tumor, based on histology, includes a metastatic undifferentiated nasopharyngeal carcinoma (NPC), other NSCLCs, metastatic melanoma, lymphomas, poorly-differentiated carcinomas, and metastatic LELC of non-pulmonary origin. LELCs have been reported as primary tumors of the breast, stomach, salivary glands, liver, thymus, uterine cervix, vagina, vulva, urinary bladder, and skin, almost all of which are EBV-associated. NPCs are also associated with EBV, and have a nearly identical histologic appearance. Therefore, patients with biopsy-confirmed pulmonary LELC should undergo workup to exclude a metastatic nasopharyngeal carcinoma.
Selected Sources
Arenas M, Gil M, Malek T, et al. Nephrotic syndrome as paraneoplastic manifestation of a primary pulmonary lymphoepithelioma-like carcinoma. CN Clinical Nephrology. 2009;72(09):206-210. doi:10.5414/cnp72206.
Chan JKI, Tai WM, Wen JM. Collision of lymphoepithelioma-like carcinoma and adenocarcinoma of the lung: a case report. The Clinical Respiratory Journal. 2015. doi:10.1111/crj.12404.
Fant W, Hong S, Chen N, He X. PD-L1 is remarkably over-expressed in EBV-associated pulmonary lymphoepithelioma-like carcinoma and related to poor disease-free survival. Oncotarget. 2015;6(32). doi:10.18632/oncotarget.5028.
Hayashi T, Haba R, Tanizawa J, et al. Cytopathologic features and differential diagnostic considerations of primary lymphoepithelioma-like carcinoma of the lung. Diagnostic Cytopathology Diagn Cytopathol. 2011;40(9):820-825. doi:10.1002/dc.21670.
He J, Shen J, Pan H, Huang J, Liang W, He J. Pulmonary lymphoepithelioma-like carcinoma: a Surveillance, Epidemiology, and End Results database analysis. Journal of Thoracic Disease. 2015;7(12):2330-2338. doi:10.3978/j.issn.2072-1439.2015.12.62.
Ho JC, Wong MP, Lam WK. Lymphoepithelioma-like carcinoma of the lung. Respirology. 2006;11(5):539-545. doi:10.1111/j.1440-1843.2006.00910.x.
Huang Y-C, Hsueh C, Ho S-Y, Liao C-Y. Lymphoepithelioma-Like Carcinoma of the Lung: An Unusual Case and Literature Review. Case Reports in Pulmonology. 2013;2013:1-4. doi:10.1155/2013/143405.
Jeong JS, Kim SR, Park SY, Chung MJ, Lee YC. A Case of Primary Pulmonary Lymphoepithelioma-like Carcinoma Misdiagnosed as Adenocarcinoma. Tuberc Respir Dis Tuberculosis and Respiratory Diseases. 2013;75(4):170. doi:10.4046/trd.2013.75.4.170.
Kawaguchi Y, Fujita T, Hanaoka J. Spontaneous Regression of Pulmonary Lymphoepithelioma-Like Carcinoma. The Annals of Thoracic Surgery. 2015;99(6):2197-2199. doi:10.1016/j.athoracsur.2014.07.088.
Liang Y, Shen C, Che G, Luo F. Primary pulmonary lymphoepithelioma-like carcinoma initially diagnosed as squamous metaplasia: A case report and literature review. Oncology Letters Oncol Lett. 2015. doi:10.3892/ol.2015.2975.
Liang Y, Wang L, Zhu Y, et al. Primary pulmonary lymphoepithelioma-like carcinoma. Cancer. 2012;118(19):4748-4758. doi:10.1002/cncr.27452.
Rossi G, Mengoli MC, Cavazza A, et al. Large cell carcinoma of the lung: clinically oriented classification integrating immunohistochemistry and molecular biology. Virchows Archiv Virchows Arch. 2013;464(1):61-68. doi:10.1007/s00428-013-1501-6.
Shen DHY, Cheng C-Y, Lin L-F, Gao H-W, Cheng Y-L, Chen C-Y. Conversion From FDG-Negative to -Positive During Follow-Up in a Rare Case of Pulmonary Lymphoepithelioma-Like Carcinoma. Clinical Nuclear Medicine. 2012;37(7):679-681. doi:10.1097/rlu.0b013e31823ea953.
Tanaka S, Chen F, Date H. Pulmonary lymphoepithelioma-like carcinoma with rapid progression. General Thoracic and Cardiovascular Surgery Gen Thorac Cardiovasc Surg. 2012;60(3):164-167. doi:10.1007/s11748-011-0789-x.
Wang L, Jiang L, Li P-F, et al. Positive expression of programmed death ligand-1 correlates with superior outcomes and might be a therapeutic target in primary pulmonary lymphoepithelioma-like carcinoma. OTT OncoTargets and Therapy. 2015:1451. doi:10.2147/ott.s84234.
Wang L, Jiang L, Li P-F, et al. Positive expression of programmed death ligand-1 correlates with superior outcomes and might be a therapeutic target in primary pulmonary lymphoepithelioma-like carcinoma. OTT OncoTargets and Therapy. 2015:1451. doi:10.2147/ott.s84234.
Wang L, Long W, Li P-F, Lin Y-B, Liang Y. An Elevated Peripheral Blood Monocyte-to-Lymphocyte Ratio Predicts Poor Prognosis in Patients with Primary Pulmonary Lymphoepithelioma-Like Carcinoma. PLOS ONE PLoS ONE. 2015;10(5). doi:10.1371/journal.pone.0126269.
Wang L, Long W, Li P-F, Lin Y-B, Liang Y. An Elevated Peripheral Blood Monocyte-to-Lymphocyte Ratio Predicts Poor Prognosis in Patients with Primary Pulmonary Lymphoepithelioma-Like Carcinoma. PLOS ONE PLoS ONE. 2015;10(5). doi:10.1371/journal.pone.0126269.
Yan G, Wei Z, Hua D, Guihua W, Xiuhua F. Inappropriate antidiuretic hormone secretion in a patient with pulmonary lymphoepithelioma like carcinoma. Chinese Medical Journal. 2014;127(14). doi:10.3760/cma.j.issn.0366-6999.20140434.
Yener NA, Balikci A, Cubuk R, Midi A, Orki A, Topkaya AE. Primary Lymphoepithelioma-Like Carcinoma of the Lung: Report of a Rare Case and Review of the Literature. TJPATH Turkish Journal of Pathology. 2012;28(3):286. doi:10.5146/tjpath.2012.01139.

 Meet our Residency Program Director
Meet our Residency Program Director
