Fomina Lab
Mechanisms Regulating Intracellular Ca2+ Dynamics
Ca2+ is a universal and versatile second messenger that regulates a large variety of cellular responses in all cell types. In T lymphocytes, spatio/temporally heterogeneous Ca2+ signals are triggered by stimulation of T cell receptor (TCR) and/or accessory molecules with an antigen. Elevation in cytosolic Ca2+ concentration ([Ca2+]i) is a major signaling pathway that connects cell surface receptors on T cells to the nucleus and is a critical regulator of T cell activation, differentiation, and survival. A sustained or oscillatory elevation in [Ca2+]i following TCR engagement is required to drive many events of lymphocyte activation, including the activation of transcription factors, modulation of cytoskeletal elements and motility, production and release of cytokines, and cell proliferation. Diminished [Ca2+]i signaling results in impaired T cell activation and, consequently, development of severe immunodeficiency. Therefore, cellular and molecular mechanisms regulating [Ca2+]i signaling in T cells at different activation states are important to elucidate.
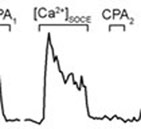
It is well established that Ca2+ release from inositol 1,4,5-trisphosphate receptors (IP3R) is an early event following TCR stimulation with antigen, which subsequently triggers activation of store-operated Ca2+ entry (SOCE). Strong TCR stimulation may also stimulate Ca2+ release from the ryanodine receptor (RyR) in a cyclic ADP ribose-dependent manner. Nevertheless, due to the limited capacity of the store, the intracellular Ca2+-release channels are believed to play a minor role in maintaining the sustained elevation of [Ca2+]i, which is critically required for acquisition of T cell effector functions. The plasmalemmal store-operated Ca2+ channels, termed Ca2+ release-activated Ca2+ (CRAC) channels are believed to be a single mechanism solely responsible for sustained [Ca2+]i elevation in T cells (Fig 1 - coming soon). However, we have found the evidence that IP3R and/or RyR control the [Ca2+]i dynamics in T cells during the SOCE by regulating Ca2+ retention within the store (Fig. 2 - coming soon). PubMed Link.
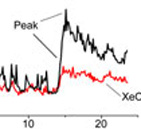
Using Fura-2 fluorescence Ca2+ indicator to monitor [Ca2+]i dynamics (Movie) during the sequential store depletion with cyclopiazonic acid, a reversible SERCA blocker, we found that Ca2+ sequestration into the store during SOCE activation occurred without global [Ca2+]i elevation, whereas Ca2+ appearance in the cytosol correlated with the store depletion Figure 3. Furthermore, the IP3R and RyR blockers xestospongin C (XeC) or ryanodine (Ry) respectively inhibited [Ca2+]i elevation upon SOCE activation in parallel with enhancement of Ca2+ retention within the store Figure 4. Taken together, these data indicate that Ca2+ sequestrating into the store occurs prior to the Ca2+ appearance in the cytosol and that the release from the IP3R and/or RyR that occurs in parallel with SOCE is the major determinant in the shaping of [Ca2+]i signaling in T cells.
The importance of intracellular Ca2+ release channels for T cell functioning was underscored by our findings that inhibition of IP3R or RyR significantly downregulated T cell proliferation and interleukin 2 production. Thus, our studies revealed a new aspect of [Ca2+]i signaling in human T cells, that is SOCE-dependent Ca2+ release via IP3R and/or RyR and identified the IP3R and RyR as potential targets for immunosuppression.





 Make a donation using our secure online system.
Make a donation using our secure online system.