The Hartwell Foundation selects two UC Davis faculty for Individual Biomedical Research Awards
Awards will fund innovative biomedical research on children’s gastric and neurodevelopmental conditions
Two UC Davis School of Medicine faculty members are among ten scientists selected to receive the 2022 Individual Biomedical Research Awards from The Hartwell Foundation. The Hartwell competition funds early-stage, innovative and cutting-edge biomedical research to benefit children in the United States.
The awardees for this year are:
- Geoanna M. Bautista, assistant professor of pediatrics in the Division of Neonatology at UC Davis Children's Hospital, for her research on intestinal inflammation and dysmotility in babies with gastroschisis.
- Roy Ben-Shalom, assistant professor of neurology and faculty at the MIND Institute, for his work on predicting the excitability of brain neuronal circuits in neurodevelopmental conditions.
Bautista and Ben-Shalom will each get $100,000 per year for three years. In addition, the Foundation is funding two UC Davis postdoctoral researchers at $50,000 per year each over two years. These researchers will pursue specialized training in biomedical research.
“We are very proud of Dr. Bautista and Dr. Ben-Shalom for being selected for this prestigious award,” said Kim Barrett, vice dean for research and distinguished professor of physiology and membrane biology. “Their programs of research are of the highest caliber and have great potential to revolutionize therapies for kids with neurodevelopmental disabilities or those born with gastroschisis.”
Intrinsic excitability of brain neuronal circuits
Neuronal electrical signaling is an important component of the brain’s circuitry. The signals generated by the movement of charged ions across the membrane can either excite or inhibit neuronal activity.

“During brain development, the electrical signals sent by individual neurons determine the connections formed with other neurons. These connections establish the neuronal pathways that make up the brain's circuitry,” Ben-Shalom explained.
Channelopathies, or mutations in ion channels, can disrupt the balance between excitatory and inhibitory neuronal activation. They lead to impaired brain connectivity and abnormal neuronal activity patterns. This excitatory-inhibitory imbalance is a hallmark of neurodevelopmental disabilities, particularly autism and childhood epilepsy.
“The mechanisms by which channelopathies lead to neurodevelopmental disabilities are poorly understood,” Ben-Shalom said. “By simulating how mutations in ion channels alter neuronal activity, we can gain insights into the underlying mechanisms of these conditions and identify potential therapeutic strategies."
According to Ben-Shalom, therapies for millions of children are designed by trial and error and based on clinical history, instead of personalized interventions specific to the child and the condition. He is developing a model that simulates changes in neuronal activity caused by alterations in ion channel function. The model will be optimized based on data from cells expressing specific channel mutations and recording the electrical signals directly from the affected channels.
“A key innovative factor of the model is in understanding how specific mutations affect electrical signaling, disrupt normal neuronal circuitry, and contribute to neurodevelopmental disabilities,” Ben-Shalom explained. “Most importantly, the model can be used to simulate the effects of treatment and inform the selection of the most effective therapy for each channel mutation.”
If successful, the simulation model will enable a better understanding of the mechanisms underlying some conditions. This will allow providers to choose specific, effective therapies to restore proper neuronal function.
A paradigm shift in understanding gastroschisis
Gastroschisis is a complex and costly birth condition that has doubled in the United States in the last two decades. A fetus with this condition develops an opening in the abdomen through which their intestines spill out into the amniotic fluid-filled uterus.
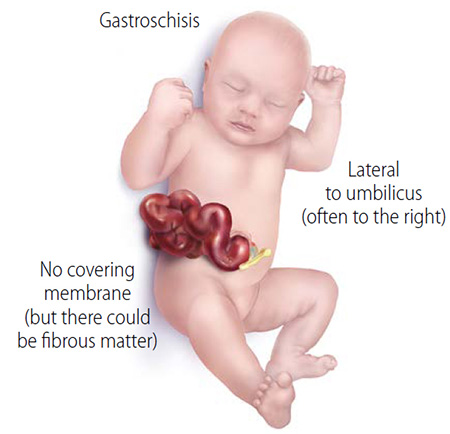
“The unprotected intestine becomes irritated, causing it to swell, shorten and get inflamed. This inflammation then leads to problems with normal bowel function after birth that can last months. Some babies may need to have part of their intestines removed, leading to short bowel syndrome and life-long challenges,” Bautista said.
Previous efforts were focused on decreasing inflammatory mediators found in the amniotic fluid of gastroschisis patients.
“These efforts have failed to show any benefit, likely due to the persistent activation of inflammation not being adequately targeted,” Bautista explained.
By applying a unique combination of cutting-edge technologies and molecular tools, Bautista aims to examine a novel pathway in gastroschisis. Her work represents a paradigm shift in thinking about the source of inflammation from the amniotic fluid to the activation of a mechanosensitive signaling pathway known as Piezo1.
“With its role in the innate immunity, inflammatory responses, and gut motility, Piezo1 may be the missing link in our understanding of gastroschisis-related complications,” Bautista said.

In her research, Bautista will use human specimens, rodent models, and a fetal gastroschisis lamb model uniquely available at UC Davis. She aims to build the foundation to develop a novel therapeutic target modulating gastroschisis-related intestinal inflammation and dysmotility (GRIID).
If successful, an entirely new, minimally invasive therapeutic strategy for GRIID may be possible, drastically improving the quality of life for children with gastroschisis.
Bautista also recently received Children’s Miracle Network grant and the Clinical and Translational Science Center KL2 award.
The Hartwell Foundation criteria for award selection
In the Hartwell competition, all nominees submit a detailed research proposal, are personally interviewed, and formally present their proposed research. The Foundation considers multiple criteria, including:
- The nature of the proposed innovation,
- The extent to which a strategic or translational approach might promote rapid clinical application of research results,
- The supportive role and extent of collaboration in the proposed research,
- The institutional commitment to provide encouragement and technical support to the investigator,
- The extent to which funding the investigator will make a difference.
On April 15, The Hartwell Foundation also announced that UC Davis is one of its 2023 Top Ten Centers of Biomedical Research. In selecting each research center of excellence, The Hartwell Foundation considers the shared values the institution has with the Foundation relating to children's health, the presence of an associated medical school and biomedical engineering program, and the quality and scope of ongoing biomedical research.
Related Stories:
Award supports cutting-edge biomedical research to benefit children with cerebral palsy