Novel study investigates cell therapy to treat swallowing disorders
UC Davis Health researchers developing therapy that uses muscle stem cells to treat patients with swallowing difficulties
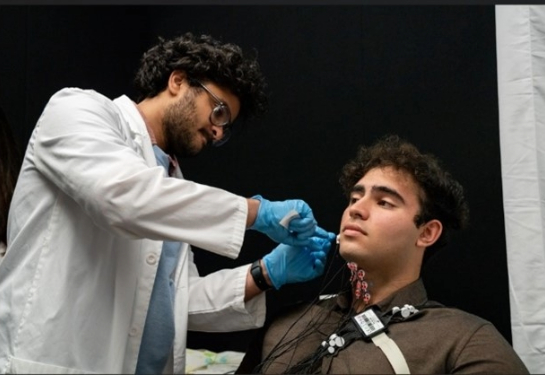
UC Davis Health researchers have launched a groundbreaking study utilizing cell therapy to increase tongue strength for patients with swallowing difficulties known as dysphagia. The project is funded by an $11 million grant from the California Institute for Regenerative Medicine (CIRM).

Researchers will take autologous muscle derived progenitor cells (AMDCs) from a biopsy of the patient’s thigh muscle and inject the cells into the patient’s tongue. The cells will fuse with existing muscle fibers to increase tongue strength and ability to swallow.
“For this study, we are developing a therapeutic approach using autologous muscle derived progenitor cells to see if there are any benefits to swallowing function for patients with dysphagia,” explained Peter Belafsky, director of the UC Davis Health Center for Voice and Swallowing and the principal investigator of the study. “This therapy could be a game-changer for our patients who suffer from difficulty swallowing, many of whom are cancer survivors living with the consequences of radiation toxicity.”
Using autologous muscle derived progenitor cells for a therapeutic approach
AMDCs are a mix of two different muscle-specific progenitor cell types called myoblasts and myocytes. They are derived from patient muscle biopsies.
AMDCs are used in a novel cell therapy developed by Cook MyoSite Inc. Researchers isolate and differentiate the muscle stem cells, called satellite cells.
For this study, the research team will get a biopsy of a patient’s thigh muscle. They will immediately ship it to Cook Myosite to extract AMDCs and expand the cell population to over 300 million per patient.
Patients receiving the treatment will have two 150 million AMDCs shots injected into the base of their tongue, spaced 4-6 weeks apart. When injected, AMDCs can fuse into the tongue and the muscle fibers. It is expected that AMDCs will increase contractile strength and improve the ability to swallow.
“The initial results of our Phase I trial indicated that AMDCs injected into the tongue of our patients with dysphagia were safe and significantly increased tongue strength,” said Belafsky.
An unmet medical need
Each year, about one in 25 adults will experience a swallowing problem in the United States. Many of these individuals have received treatment for head and neck cancer, which can damage the muscles responsible for a safe and effective swallow. Currently, there are no effective treatments to improve swallowing.
This therapy could be a potential game-changer for our patients who suffer from difficulty swallowing, many of whom are cancer survivors living with the consequences of radiation toxicity.” —Peter Belafsky
“This is a struggling patient population that currently doesn't have many good options to help them,” explained Belafsky. “They can experience dramatic deficits in both speech and swallowing that may lead to malnutrition, dehydration, social isolation, depression, aspiration pneumonia, pulmonary abscess and even death.”
The results of this clinical trial will potentially benefit millions of patients with swallowing disorders. They could eventually lead to the development of this product into treatments for other muscle disorders.
“If successful, we anticipate an accelerated commercialization of the AMDC product candidate with Cook MyoSite to address this unmet clinical need,” explained Belafsky. “The potential implications of this work are simply profound.”
Study seeking patients struggling with swallowing disorders
The researchers are working to identify and recruit patients who:
- are 18 years of age or older,
- have moderate symptoms of tongue-related swallowing difficulties following surgery, chemo or radiotherapy for head and neck cancer,
- have completed treatment over 24 months before enrollment,
- have problems swallowing that have not improved following current therapies.
The study will enroll 62 patients at two clinical sites (UC Davis and UC San Francisco). The patients will receive either two intramuscular injections of 150 million AMDC cells into the tongue or placebo injections. They will be followed for 24 months post-treatment.
“We are recruiting patients who are highly motivated to be part of a study that could lead to a cure of this ailment,” said Belafsky.
Additional investigators for the study include Clark Rosen, director of the UC San Francisco Voice and Swallowing Center and chief of the laryngology division.
For more information, contact study coordinator Randev Sandhu at 916-734-2863 or HS-ENTStemCell@ucdavis.edu.