New tumor models provide insights into deadly sarcomas
Sarcomas are highly metastatic soft tissue and bone cancers and are often difficult to treat. Scientists have had trouble studying these cancers because they have lacked good research models. But that may be changing, thanks to new research by investigators at UC Davis Comprehensive Cancer Center and UCLA Health’s Jonsson Comprehensive Cancer Center.
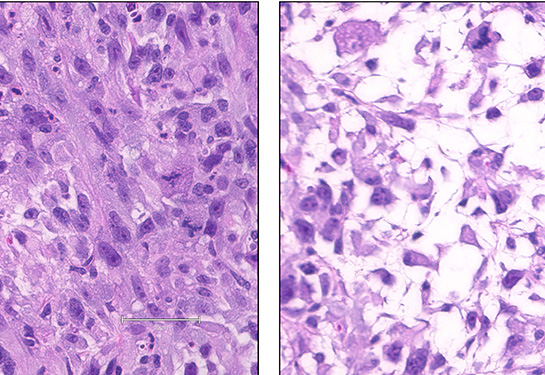
In a study published in the journal Clinical Cancer Research, the teams report they have manipulated mesenchymal stem cells to create four different sarcoma models. This versatile platform has already identified potential therapeutic targets and could eventually lead to new treatments.
“I'm excited about this model because we can take the same starting material, mesenchymal stem cells, and turn them into four different sarcoma types,” said Janai Carr-Ascher, assistant professor in the Division of Hematology and Oncology and senior author of the study. “This gives us an incredible tool, allowing us, for the first time, to understand the similarities and differences between these tumors, to learn how they develop, evolve and metastasize.”
As part of the highly collaborative effort, Carr-Ascher worked closely with Thomas Graeber, professor of molecular and medical pharmacology in UCLA’s Broad Stem Cell Research Center and others in his lab, including graduate student Jack Freeland. He and UC Davis graduate student Maria Muñoz were co-first authors.
Sarcomas have been difficult to treat because they’re often diagnosed late and are wildly diverse — there are around 70 different types. By contrast, there have been very few sarcoma models to study. The most common sarcomas behave in radically different ways, and because they are so poorly understood, clinicians have been limited to just a few therapies. Carr-Ascher and colleagues believe these new models could help generate more precise treatment options.
“Once we understand the distinctions that drive some cells toward bone cancer and others toward various soft tissue cancers, we can begin to interrogate the disease,” said Carr-Ascher. “Hopefully, once we know how it's put together, we can understand how to take it apart.”
Mesenchymal stem cells are primitive cells that differentiate into bone, cartilage, muscle and fat. In 2017, Carr-Ascher and colleagues began studying the genetic signals that influence mesenchymal cells to become different sarcomas.
Once we understand the distinctions that drive some cells toward bone cancer and others toward various soft tissue cancers, we can begin to interrogate the disease. Hopefully, once we know how it's put together, we can understand how to take it apart.”—Janai Carr-Ascher, UC Davis Comprehensive Cancer Center oncologist
The team started with sarcoma data from The Cancer Genome Atlas. They found that two well-known oncogenes (YAP1 and KRAS) direct mesenchymal cells to become undifferentiated pleomorphic sarcoma and myxofibrosarcoma, two of the most common soft tissue cancers in adults. Two other oncogenes (CDK4 and PIK3CA) produce leiomyosarcoma (smooth muscle cancer) and osteosarcoma (bone cancer), although less consistently.
These results highlight the model’s versatility. While it is no easy feat to identify the genetic signals that differentiate a mesenchymal stem cell into any specific sarcoma, this model provides a template to test the roles of different proteins in this process. Once the appropriate oncogenes are identified, it takes scientists around a week to create the viral delivery system to get them into stem cells. With this technology, researchers can potentially create models for many, if not all, sarcomas.
Taking the research a step further, the team treated the YAP1 tumors with existing anti-cancer agents, which reduced cell viability. Ultimately, these models could become indispensable tools to help develop more advanced anti-sarcoma therapies.
“We were able to take our model, overlap it with the human disease and use this analysis to identify new therapeutics to test,” Graeber said. “Furthermore, these models are genomically unstable, just like human sarcomas, and understanding why their genomes are so unstable is a critical topic in sarcoma research. Increased genomic instability has been linked to aggressive tumor growth and drug resistance, and this is an area where we need advances.”
The team will continue to interrogate these new sarcoma models. They are particularly interested in the mechanisms that drive metastases. In addition, there may be other existing therapies that could be repurposed to treat sarcomas.
“Based on this data and what we've seen in The Cancer Genome Atlas, we believe YAP1, which is part of a key signaling pathway, is super important in sarcomas,” said Carr-Ascher. “There are related inhibitors in clinical trials right now for other tumor types. If we can show the pathway’s importance and identify useful combination therapies, that could have major translational impact.”
“Dr. Carr-Ascher and her team have developed highly sophisticated mouse models of complex genotype sarcomas. Such work brings hope for the discovery of novel therapeutic targets that can ultimately save lives,” said R. Lor Randall who is chair of Orthopaedic Surgery and The David Linn Chair in Orthopaedic Surgery.
This research was funded by National Institutes of Health, the Doris Duke Charitable Foundation, Burroughs Wellcome Fund, Alan B. Slifka Foundation, W.M. Keck Foundation, the UCLA Broad Stem Cell Research Center, and other organizations.
UC Davis Comprehensive Cancer Center
UC Davis Comprehensive Cancer Center is the only National Cancer Institute-designated center serving the Central Valley and inland Northern California, a region of more than 6 million people. Its specialists provide compassionate, comprehensive care for more than 100,000 adults and children every year and access to more than 200 active clinical trials at any given time. Its innovative research program engages more than 240 scientists at UC Davis who work collaboratively to advance discovery of new tools to diagnose and treat cancer. Patients have access to leading-edge care, including immunotherapy and other targeted treatments. Its Office of Community Outreach and Engagement addresses disparities in cancer outcomes across diverse populations, and the cancer center provides comprehensive education and workforce development programs for the next generation of clinicians and scientists. For more information, visit cancer.ucdavis.edu.