Researchers Identify Protein Linked to Metastasis in Pancreatic Cancer
Discovery Could Advance Life-Saving Therapies
Pancreatic cancer is the No. 3 cause of cancer-related deaths in the United States, and only 12% of patients survive five years after being diagnosed. Severe pancreatic cancer is associated with metastasis, and it is this spread of secondary tumors that usually causes death, but little is known about the molecular mechanisms that drive metastasis.
In a study published Dec. 18 in Advanced Science, researchers from the University of California, Davis showed that abnormal expression of the protein Engrailed-1(EN1) promotes pancreatic cancer progression and metastasis in vitro and in mouse models. The team also found that elevated EN1 was associated with severe, metastatic pancreatic cancer in human patients, which suggests that EN1might make a good target for pancreatic cancer therapies.
“We identified a novel epigenetic factor that can contribute to metastasis in pancreatic cancer, which is one of the most challenging cancers to treat,” said Chang-Il Hwang, an assistant professor in the UC Davis Department of Microbiology and Molecular Genetics and a senior author on the paper. “A better understanding of these mechanisms would allow us to identify potential targets and improve patient survival.”
Uncovering a main actor in pancreatic metastasis
Metastasis is an important component of pancreatic cancer progression, but researchers have not been able to identify genetic mutations responsible for it. For this reason, Hwang thought that nongenetic factors, such as epigenetic changes or altered protein production, might be at play. His team previously identified several transcription factors — proteins that control the production of other proteins — that are elevated in pancreatic cancers that have undergone metastasis compared to primary tumors.
One of these proteins, EN1, is essential for the survival of neurons during development and is not usually produced in adult pancreatic cells. EN1 has been shown to promote aggressive forms of breast cancer, and it is also associated with poor prognosis in other cancers, including glioblastoma and salivary gland adenoid cystic carcinoma, but its role in pancreatic cancer had not previously been described.
The researchers tested whether inhibiting EN1 or ramping up its expression impacted the growth and survival of pancreatic cancer “organoids” — three-dimensional clumps of lab-grown tissue. They found that, without EN1, pancreatic cancer cells were less likely to survive and divide, but adding extra EN1 increased the tumors’ survival. Furthermore, when the researchers genetically modified mouse pancreatic cancer cell lines so that they produced more EN1 than usual, the cells showed increased rates of cell invasion and migration, key features of metastasis.
“It’s very clear that EN1 is a really important factor behind the aggressiveness of pancreatic cancer,” said first author Jihao (Reno) Xu, a doctoral candidate in the Biochemistry, Molecular, Cellular and Development Biology graduate group. “When we take the tumor cells and make them overexpress EN1, they become more metastatic and aggressive, and when we knock it down, they become less metastatic.”
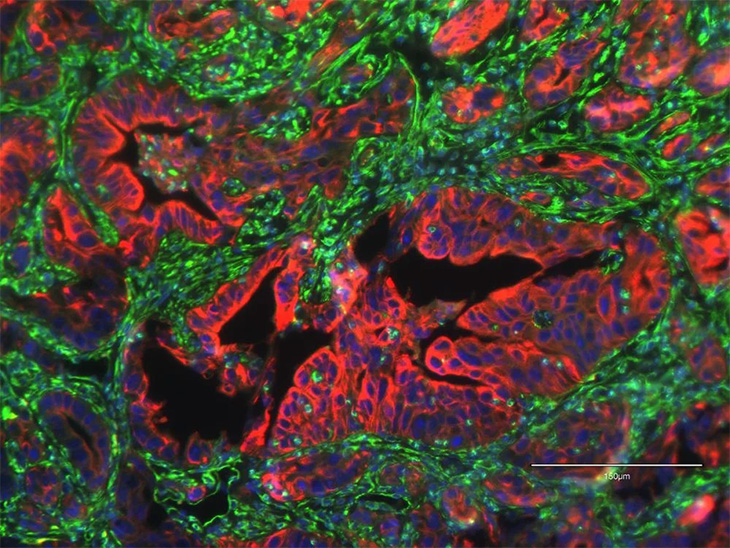
By analyzing publicly available patient databases, the researchers also showed that EN1 is important for prognosis in human pancreatic cancer. They found that EN1 levels were elevated in a subset of patients with advanced pancreatic cancer, and that patients with elevated EN1 tended to have worse prognoses.
“Patients with high levels of EN1 have shorter survival times, which suggests that it is contributing to the aggressiveness of pancreatic cancer,” said Hwang.
Now, Hwang, Xu and their colleagues are working on ways to translate their findings into the clinic by testing different ways to target EN1. They also plan to continue investigating other nongenetic factors that might contribute to pancreatic cancer progression.
“Ultimately, we want to identify new therapeutic strategies to tackle this disease,” Xu said.
Additional authors on the paper are: at UC Davis, EunJung Lee, Keely Y. Ji, Omar W. Younis and Alexander D. Borowsky; Jae-Seok Roe, Yonsei University; Claudia Tonelli, Tim D.D. Somerville, Melissa Yao, Joseph P. Milazzo, Herve Tiriac, Youngkyu Park, Christopher R. Vakoc and David A. Tuveson, Cold Spring Harbor Laboratory; Ania M. Kolarzyk and Esak Lee, Cornell University; Jean L. Grem, Audrey J. Lazenby, James A. Grunkemeyer and Michael A. Hollingsworth, University of Nebraska Medical Center.
The work was supported by the UC Davis Comprehensive Cancer Center Pilot Grant and the National Institutes of Health.
UC Davis Comprehensive Cancer Center
UC Davis Comprehensive Cancer Center is the only National Cancer Institute-designated center serving the Central Valley and inland Northern California, a region of more than 6 million people. Its specialists provide compassionate, comprehensive care for more than 100,000 adults and children every year and access to more than 200 active clinical trials at any given time. Its innovative research program engages more than 240 scientists at UC Davis who work collaboratively to advance discovery of new tools to diagnose and treat cancer. Patients have access to leading-edge care, including immunotherapy and other targeted treatments. Its Office of Community Outreach and Engagement addresses disparities in cancer outcomes across diverse populations, and the cancer center provides comprehensive education and workforce development programs for the next generation of clinicians and scientists. For more information, visit cancer.ucdavis.edu.






