Residency Program - Case of the Month
September 2009 Final Diagnosis - Presented by Heidi Jess, M.D.
Answer:
Angiosarcoma of the soft tissue
Histologic description:
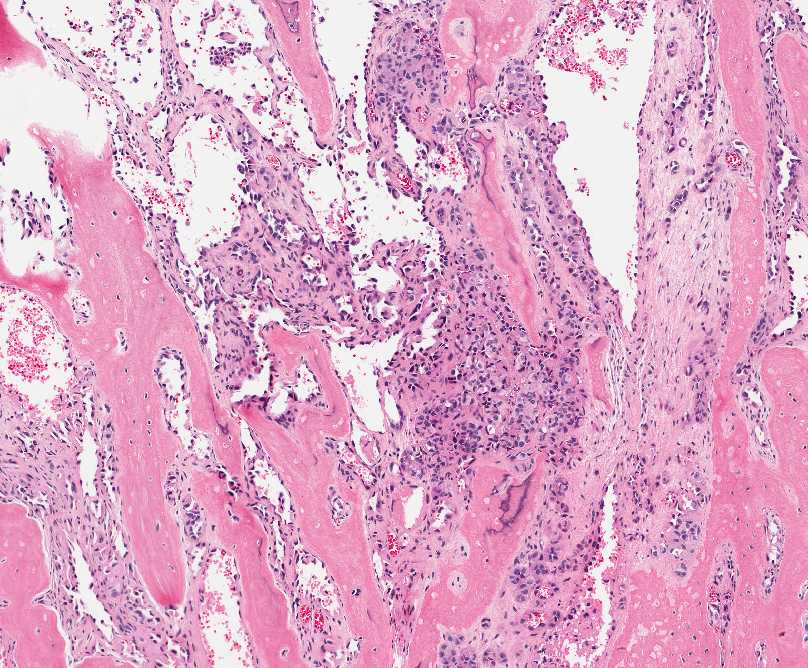
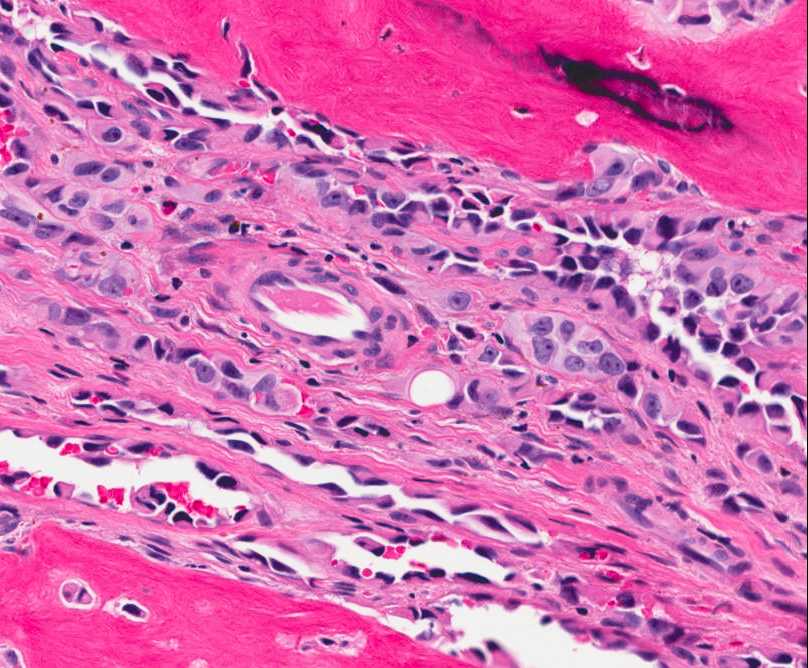
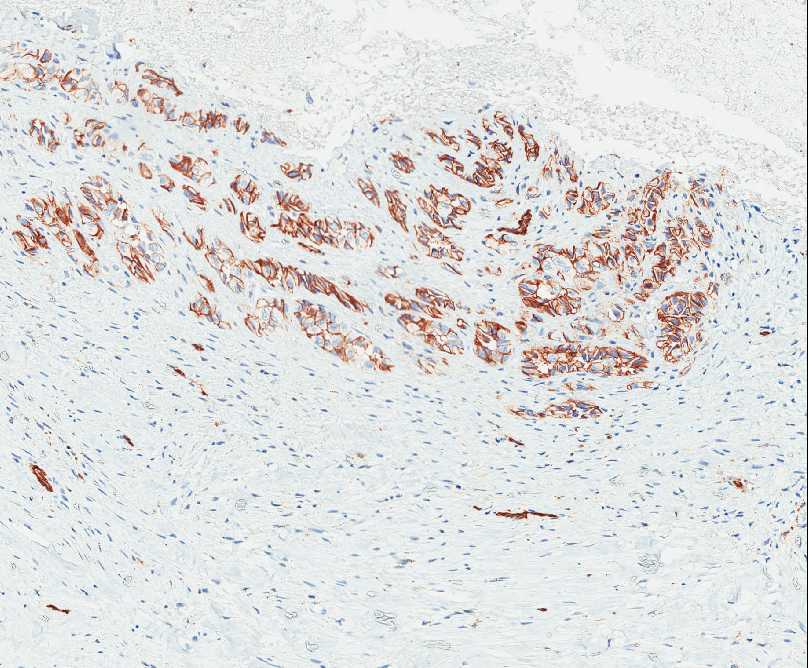
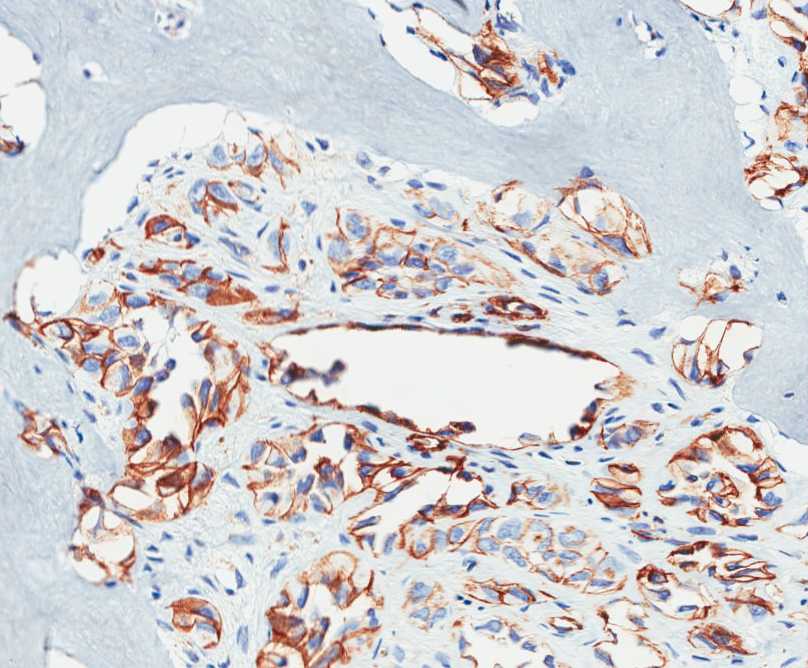
The tumor is composed of two distinct morphologic areas (Figure 1). One area is composed of nests of pleomorphic cells with abundant eosinophilic cytoplasm, irregular nuclear membranes and hyperchromatic nuclei (Figure 4). Some of these cells contain prominent nucleoli. These cells also form irregular vascular spaces and are discohesive in the center with a hobnail appearance (Figure 2-3,5). Mitoses are rare. Occasional cells with cytoplasmic vacuoles are seen. The other area is composed of abundant osteoid which is lined by osteoblasts with abundant amphophilic cytoplasm and eccentric hyperchromatic nuclei (Figure 2). The lacunar spaces contain small osteocytes (Figure 3). The areas of osteoid formation appear to be reactive in nature. The neoplastic cells express CD31 (Figure 6-7) and CD34 immunohistochemical markers.
Discussion:
Angiosarcoma represents less than 1% of all soft tissue sarcomas and most commonly occurs within the cutaneous [2-4]. Less than one quarter of angiosarcomas occur within the deep soft tissue, most commonly in the deep muscles of the lower extremities [1-2]. Other sites of origin include the liver, breast, spleen, upper extremities, trunk and head and neck. The clinical presentation and behavior differs depending on the site of origin. Subsequently, angiosarcomas can be divided into several groups: cutaneous angiosarcoma, angiosarcoma associated with lymphedema, angiosarcoma of the breast, radiation-induced angiosarcoma and angiosarcoma of deep soft tissue [2]. Rarely angiosarcoma can develop adjacent to foreign material or past traumatic site, in other tumors, or in association with genetic syndromes such as Neurofibromatosis Type 1, Kippel-Trenaunay and Maffucci syndromes [1-2,5]. Angiosarcomas of the deep soft tissue can occur at any age [2]. Most patients are asymptomatic or may present with an enlarging mass or a chronic hematoma [1-2].
Typically these lesions are ill-defined, sometimes multinodular, hemorrhagic masses that range in size from a few to several centimeters. Microscopically, the cells may vary from spindle to epitheloid in appearance with the majority of the tumor typically comprised of neoplastic epitheloid cells [1-2]. The epitheloid cells have abundant eosinophilic cytoplasm and large vesicular nuclei. Some cells may contain intracytoplasmic lumens [2]. The cells are arranged in sheets, nests, cords or rudimentary vascular channels and dilated vascular spaces [1,5]. The vascular channels are irregular in shape and freely anastomosis with one another. Extensive hemorrhage is a characteristic feature which may lead to a misdiagnosis of a chronic hematoma. The cells typically have a high nuclear grade and the mitotic rate may vary [1]. Metaplastic bone formation within angiosarcoma has not been reported in the literature, and which is a very unusual characteristic of the current case.
For poorly differentiated angiosarcoma, immunohistochemical staining is an important adjunctive. Angiosarcomas will typically express the usual vascular antigens such as von Willebrand factor, CD31, and CD34. CD31 has the highest sensitivity and specificity. Von Willebrand factor has a high specificity but a low sensitivity [1]. Approximately one third of angiosarcomas will express cytokeratin [1-2]. Laminin and type IV collagen can be detected around the neoplastic channels [1].
Most angiosarcomas show complex cytogenetic aberrations and no consistent recurring chromosomal abnormality has been identified. Angiosarcoma arising in a cavernous hemangioma will typically show trisomy 5 and loss of the Y chromosome [1].
The differential diagnosis for angiosarcoma of the soft tissue includes high grade epithelioid hemangioendothelioma; however, given the degree of cytologic atypia, occasional mitotic figures and large vascular spaces, epithelioid hemangioendothelioma is less likely. Kaposiform hemangioendothelioma occurs nearly exclusively during childhood and teenage years and also lacks cytologic atypia and mitotic figures. The differential may also include epitheloid sarcoma. These tumors will not express CD31. Given the unusual amount of metaplastic bone formation in the current case, osteosarcoma can be considered in the differential diagnosis; however, given the bland appearance of the osteoblasts and osteocytes, and the positivity of the neoplastic cells for numerous vascular markers, the diagnosis of osteosarcoma can be excluded.
Treatment consists of wide local excision. Due to the rarity of angiosarcoma, the role of radiation and chemotherpay is not well defined. Soft tissue angiosarcomas are highly aggressive tumors. One fifth of patients will develop local recurrences and one half will die within the first year after diagnosis [1]. One study reported a 5-year survival rate of 23% [4]. Poor prognostic factors include older age, retroperitoneal location, large size and high Ki-67 value (>10%) [1-2].
References:
- Christopher D.M. Fletcher, K. Krishnan Unni, Fredrik Mertens: World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon 2002.
- Sharon W. Weiss and John R. Goldblum: Enzinger and Weiss's Soft Tissue Tumors, Fourth Edition, 2001.
- Andrea T. Deyrup, Markku Miettinen, Paula E. North, Joseph D. Khoury, Mourad Tighiouart, Sheri L. Spunt, David Parham, Sharon W. Weiss, Bahig M. Shehata: Angiosarcomas Arising in the Viscera and Soft Tissue of Children and Young Adults, A Clinicopathologic Study of 15 Cases. American Journal of Surgical Pathology, Volume 33, Number 2, February 2009.
- J. Fayette, E. Martin, S. Piperno-Neumann, A. Le Cesne, C. Robert, S. Bonvalot, D. Ranchere, P. Pouillart, J. M. Coindre, J. Y. Blay: Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Annals of Oncology, Volume 18, 2007.
- Fred G. Fedok, Roger J. Levin, Mary E. Maloney, Kiran Tipirneni. Angiosarcoma: Current Review. American Journal of Otolaryngology, Volume 20, Number 4, 1999.
|
Figure 1: Tumor is composed of two distinct morphologic areas: 1) neoplastic cells forming nests and dilated vascular spaces, 2) metaplastic bone. |
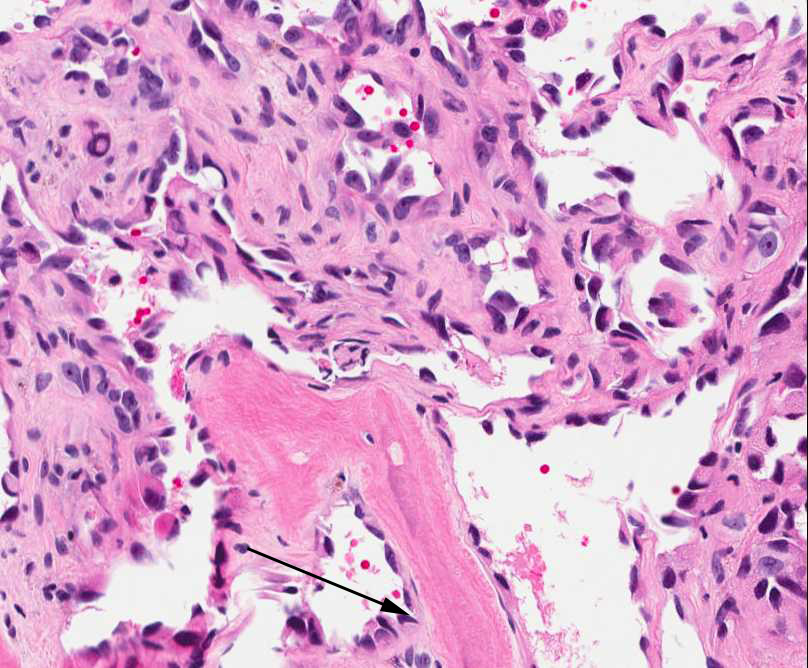
Figure 2: Reactive osteocytes are rimming the metaplastic bone (arrow). Tumor cells are forming an irregular vascular network. |
|
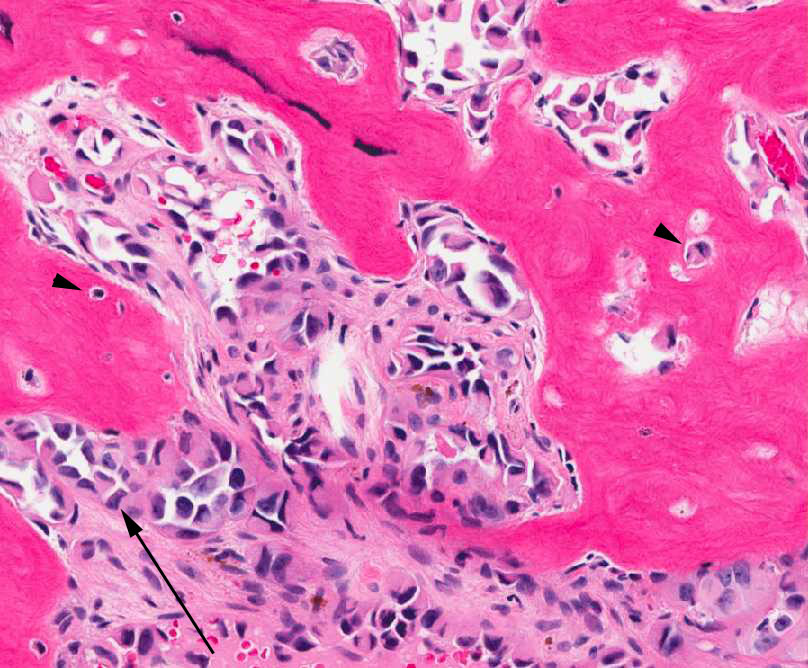
Figure 3: Tumor cells are forming a loose vascular network with areas of hobnail appearance (arrow). Reactive osteocytes are noted within the metaplastic bone (arrowhead). |
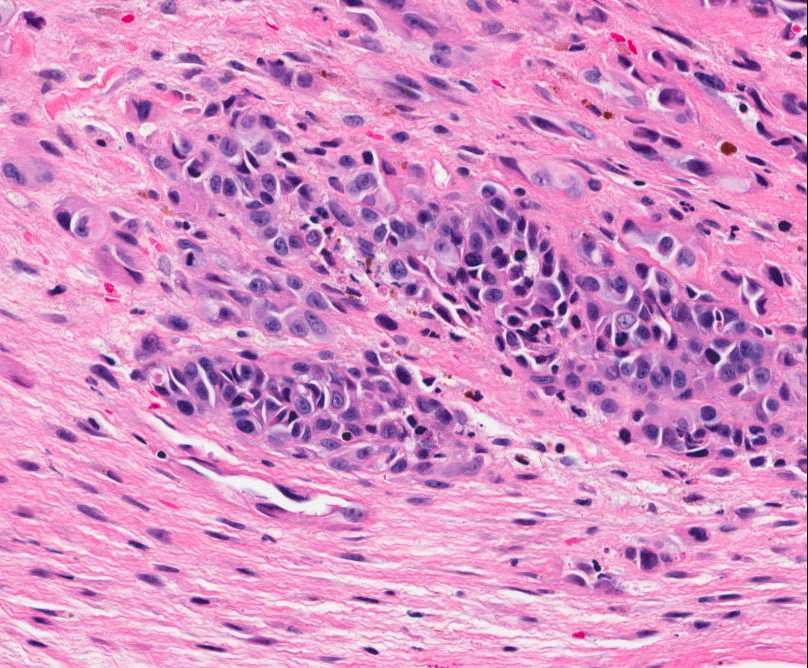
Figure 4: Nests of neoplastic cells with abundant eosinophilc cytoplasm and prominent nucleoli, surrounded by reactive fibrous tissue. |
|
Figure 5: The neoplastic cells are forming vascular spaces and nests between areas of reactive bone formation. |
Figure 6: Nests of neoplastic cells express CD31. |
|
Figure 7: The neoplastic cells express CD31. |

 Meet our Residency Program Director
Meet our Residency Program Director
