Researchers discover how some brain cells transfer material to neurons in mice
New study opens treatment possibilities for Alzheimer’s, Parkinson’s and Huntington’s diseases
Researchers at UC Davis are the first to report how a specific type of brain cells, known as oligodendrocyte-lineage cells, transfer cell material to neurons in the mouse brain. Their work provides evidence of a coordinated nuclear interaction between these cells and neurons. The study was published today in the Journal of Experimental Medicine
“This novel concept of material transfer to neurons opens new possibilities for understanding brain maturation and finding treatments for neurological conditions, such as Alzheimer’s disease, cerebral palsy, Parkinson’s and Huntington’s disease,” said corresponding author Olga Chechneva. Chechneva is an assistant project scientist at UC Davis Department of Biochemistry and Molecular Medicine and independent principal investigator in the Institute for Pediatric Regenerative Medicine at Shriners Children's Northern California.
Our knowledge about this mechanism is extremely new, and it opens many questions for understanding how neurons work and its biological relevance in many neurological disorders. This is very exciting.”—Olga Chechneva
What are oligodendrocyte-lineage cells?
Oligodendrocyte-lineage cells, also called oligodendroglia, are a type of glial cells found in the central nervous system. From birth onward, these glial cells arise to support neural circuit maturation. They are mostly known for their role in myelination - the formation of the insulating myelin sheath around nerve axons.
Satellite oligodendrocytes are a distinct type of oligodendroglia found in close contact with neuronal bodies in the gray matter of the central nervous system. They are involved in several functions, including supporting the survival of nearby neurons, regulating neurotransmitter release, and modulating synaptic activity. They have a different form and structure than the classic oligodendrocytes that produce myelin in the white matter.
“Research has mostly focused on studying the myelinating function of oligodendrocytes, while satellite oligodendrocytes and their interaction with neurons are not well understood,” Chechneva said.
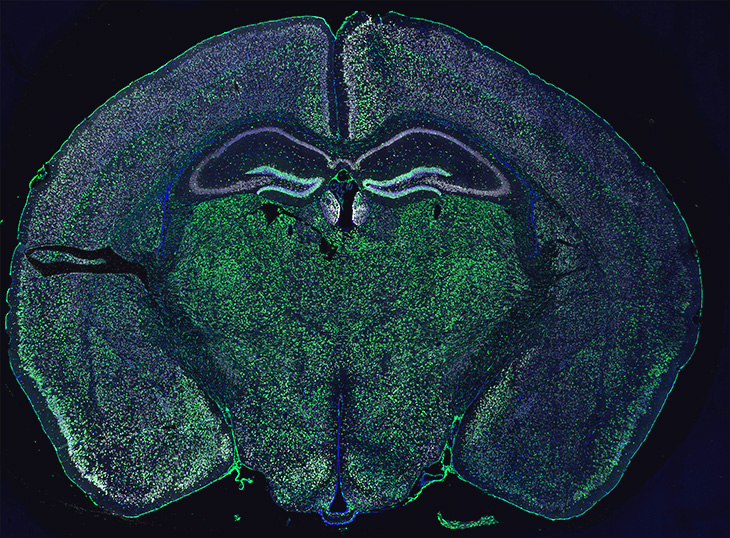
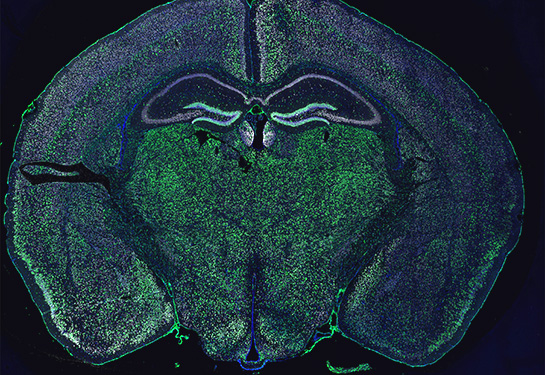
Unexpected observation makes a new discovery possible
Capturing the glia-neuron interaction started with an unexpected observation. The researchers were using special fluorescent proteins to label and track oligodendroglia in the mouse brain and spinal cord. They were surprised to find that ribosomal and nuclear reporter proteins were not only present in these cells, but also inside neurons of the mouse model.
“When something unexpected like this happens, we need to make sure it’s not an artifact,” Chechneva said. “It was puzzling why these proteins, which should only be in oligodendroglia, are also in neurons. Our team used different controls and did multiple experiments to conclude that oligodendrocytes transfer nuclear and ribosomal material to neurons.”
Open borders for material transfer
Material transfer of proteins and molecules from neuron to neuron and glia cells to neurons is critical to neuronal survival, function and recovery after injury.
Until now, there were two known ways of material transfer in the neural system. The first is tunneling nanotubes or gap junctions, which are channels that allow for direct communication between cells. The other mechanism is through the release of extracellular vesicles (small structures that contain proteins, lipids, and nucleic acids). These vesicles can transfer various molecules that can be taken up by neighboring cells.
This study is the first to capture and report on satellite oligodendrocytes found in contact with neurons that received material and with the plasma membrane between them interrupted.
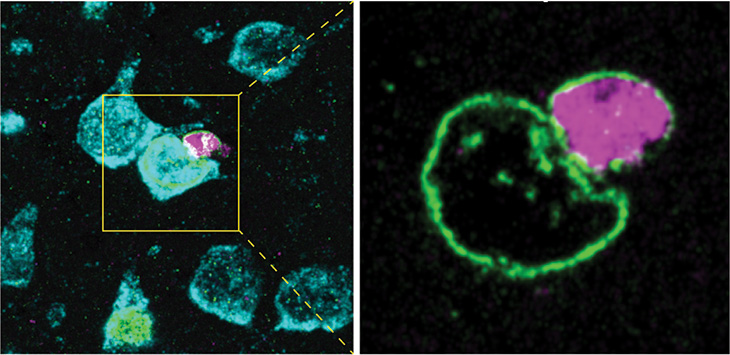
“We are looking at the possibility of an additional new mechanism where the cell can directly transfer material to another cell, in particular satellite oligodendrocyte to neuron. We could clearly see that cell nuclei from both cells come together, and the plasma membrane (the physical border between cells) was open,” Chechneva explained.
Exploring the transfer mechanism for potential therapies to neurodegenerative diseases
The study showed that oligodendroglia-neuron material transfer establishes after birth, during a critical period of brain maturation.
“The fact that this transfer process is established during postnatal development is very interesting. These are critical periods when the brain is maturing and brain circuits are formed,” Chechneva said.
The team still doesn’t know the regulatory mechanism of material transfer or its duration.
“Our knowledge about this mechanism is extremely new, and it opens many questions for understanding how neurons work and its biological relevance in many neurological disorders. This is very exciting,” Chechneva added.
The researchers also discovered that material transfer to neurons is increased during chronic neuroinflammation. They see potential in using this finding to develop better-targeted therapies for conditions caused by the accumulation of pathogenic proteins in neurons, such as in Alzheimer’s and Parkinson’s. The team’s next research goal is to determine the biological function of oligodendroglia-neuron material transfer in development and in neurodegeneration.
Florian Mayrhofer is a co-corresponding author of the study. The other authors are Angela Hanson, Manuel Navedo, Yang Xiang, Athena Soulika and Wenbin Deng.
This work has been funded by the National Institutes of Health (NIH) (grants R01HL149127, R01GM129376, R01HD087566, R01HD091325), Shriners Hospital for Children (NC-87310 and 85114-NCA) and the National Multiple Sclerosis Society (RG-1701-26770).