Developing a better diagnostic test and possible therapy for myasthenia gravis
Scientists have created a molecule that could detect and remove the pathogenic autoantibodies associated with the disease.
The National Institutes of Health’s (NIH) Blueprint MedTech program recently awarded UC Davis Health biophysical chemist Robert Fairclough a grant to develop an improved diagnostic test for myasthenia gravis, a chronic autoimmune disease.

Fairclough, professor emeritus in the Department of Neurology at the UC Davis School of Medicine, has developed a protein that could help diagnose and treat myasthenia gravis.
He has spent decades studying the acetylcholine receptor (AChR), a key player in translating critical biochemical signals from nerves to muscles.
“The chemical messenger acetylcholine, released from a nerve terminal, binds to its receptor on the muscle fiber, activating the machinery that generates muscle contractions,” Fairclough said. “It’s right at the heart of the network converting electrical/chemical signals into muscle contractions. So, losing AChR plays a fundamental role in developing myasthenia gravis.
In people with myasthenia gravis, the nerve/muscle communication system breaks down as their antibodies mistakenly attack the acetylcholine receptors, generating a loss of receptors. These pathogenic anti-AChR antibodies can alter muscle function, leading to eye muscle weakness, difficulty swallowing, shortness of breath, speech impairment, and other large muscle issues.
Developing a diagnostic
Over the years, Fairclough’s lab has dissected the acetylcholine receptor’s five subunits. They found that one specific region of the alpha subunits (the main immunogenic region or MIR) is immunologically hot. In other words, this is where the pathogenic antibodies like to strike.
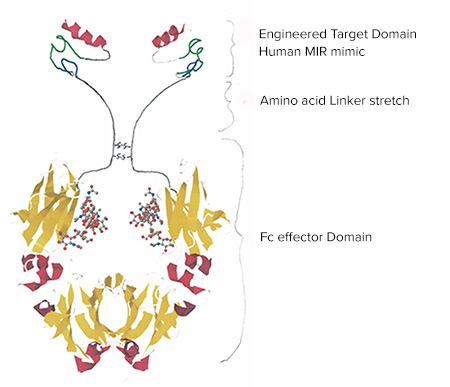
In response, the lab designed a peptide that mimics the MIR. This peptide acts as a decoy. It binds to these pathogenic autoantibodies and prevents them from attaching to the actual receptor. This binding activity offers both diagnostic and therapeutic possibilities.
On the diagnostic side, the peptide can detect MIR-directed autoantibodies, biomarkers for myasthenia gravis, in a patient’s blood serum. The NIH grant will support the development of this diagnostic against pathogenic anti-MIR antibodies.
Unlike existing tests that use radioactive isotopes to identify all the anti-AChR antibodies, Fairclough’s assay locks onto the pathogenic anti-MIR autoantibodies. This process could help physicians better determine each patient’s disease state and measure treatment outcomes.
“We believe we can use this peptide as a diagnostic test to measure the amount of these pathogenic anti-MIR antibodies in a patient’s serum,” Fairclough said. “Even more importantly, this information will help guide treatments of myasthenia gravis.”
A potential therapy
Fairclough’s peptide may have even greater potential as a therapeutic. The lab has attached the peptide to a synthetic antibody. Together, they block the anti-MIR antibodies, limiting their ability to activate the immune response. This potential biologic drug would bind to the pathogenic antibodies and red blood cells would usher the complex to the liver for clearance.
In addition, the drug is designed to bind to the memory B cells that generate the autoantibodies, targeting those cells for immune destruction. The Fairclough lab’s peptide-based diagnostic and associated therapy will not cure myasthenia gravis, but could dramatically improve patients’ quality of life.
“The biologic has a lot of advantages over traditional therapies, such as steroids and immunosuppressive drugs, which can have severe side effects,” Fairclough noted. “We could give patients a dose every couple of months to keep their anti-MIR autoantibodies under control. The diagnostic gives us the ability to measure the concentration of those antibodies in each patient, so we can determine the correct dosage and develop a precise schedule to administer the drug.”
This work is a collaboration between Fairclough and molecular biochemist Vu Trinh. Together, they also created Sierra Biopharma, a company to design and refine therapeutics for autoimmune diseases.