Robert H. Fairclough, Ph.D.
Brief Biography
 Dr. Fairclough is a Biophysical Chemist experienced with fluorescence spectroscopy, electron microscopy, X-ray scattering and diffraction. He uses these as well as immunological tools and electrophysiology in the study of ion channels and wayward auto-immune diseases.
Dr. Fairclough is a Biophysical Chemist experienced with fluorescence spectroscopy, electron microscopy, X-ray scattering and diffraction. He uses these as well as immunological tools and electrophysiology in the study of ion channels and wayward auto-immune diseases.
During graduate school at Columbia, Dr. Fairclough studied the structure and function of the E.coli ribosome using fluorescence resonance energy transfer (FRET). Then, during his postdoctoral studies at Stanford and UCSF, he applied anomalous X-ray scattering and membrane small angle X-ray diffraction to localize Ca binding sites on the Torpedo acetylcholine receptor (AChR).
His interest in the AChR continued as an assistant professor of Neurology at the U of Chicago where, with Dr. David Richman, he began a study of the location of various anti-AChR monoclonal antibody binding sites on the AChR using electron microscopy and membrane X-ray diffraction.
Dr. Fairclough moved to UC Davis as an Associate Professor in Neurology where his interest turned to mapping the components of the AChR main immunogenic region (MIR) to which 50-70% of anti-AChR Abs in MG serum are directed. He is now an Emeritus Professor.
Basic Research Overview
The neuromuscular nicotinic acetylcholine receptor (AChR) is a five subunit integral membrane protein lodged in the post-synaptic membrane of the neuromuscular junction synapse. The binding of two equivalents of ACh induces a structural change in the AChR that opens very briefly (a couple of milliseconds) a cation specific ion channel pore allowing Na+ and Ca2+ ions to flow down their electrochemical gradients across the muscle cell membrane, depolarizing the -70 mV membrane potential representing the first step of several leading to muscle contraction.
He asked “Where on the AChR is the ACh binding site?” Given the low resolution structural model of the AChR at the time (A below), and having determined that mAb 383C blocks the binding of 1 of the 2 ACh binding sites, the one at the α/γ subunit interface, he used electron microscopy and membrane small angle X-ray diffraction to localize the combining site of mAb 383C some 35Å down from the top of the AChR and at the pentagonal vertex away from the C2 axis between the donut pairs with stain exclusion (green fuzz) between the pentagons in B below. This is the location of the δ-δ disulfide between pairs of pentagons (B below). This study also established the subunit organization as αγαδβ counter-clockwise about the donut (C below), a topic in much contention.
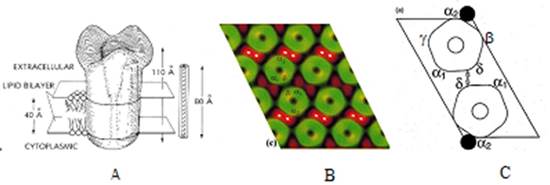
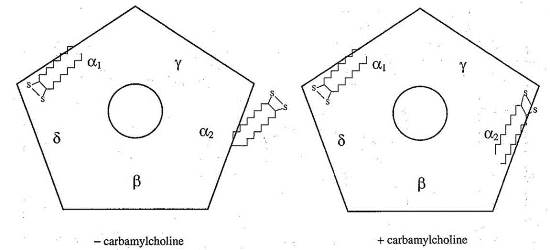
Next he asked “What is the structural change that accompanies binding of agonist to the receptor?” Using FRET, he measured the distance between alpha cys192/193 labeled with iodoacetamido-fluorescein at the α/γ agonist site (α2) and rhodamine labeled alpha bungarotoxin (R-Btx) bound to the other alpha subunit (α1) binding site, and compared the distance measured before and after adding agonist and found the distance contracts 10Å upon addition of agonist (see below).
Translational Research Overview
Having mapped the main immunogenic region (MIR) of the Torpedo AChR to three non-contiguous segments of the α subunit: α(1-12), α(65-79), and α(110-115) with mAb 132A, he designed and synthesized 39 amino acid peptide mimics of the Torpedo MIR, the rat MIR, and the human MIR and characterized their ability to bind a number of MIR directed mAbs.
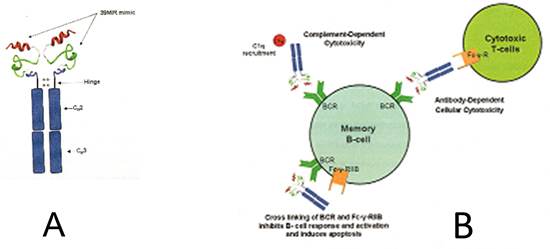
His team subsequently attached the rat mimic to the rat IgG Fcγ2b just above the hinge region, and a similar construct to the human Fc of a IgG γ1 (see A below). These are being tested for the ability to form complexes with anti-MIR Abs in myasthenic sera which represent 50-70% of the anti-AChR Abs in MG sera. The goal is to have this therapeutic agent that will adsorb pathogenic MIR directed Abs from MG patient serum and bind to and kill memory B cells with the blueprint for production of more anti-MIR Abs. Perhaps with sufficient craft the disease can be cured by an antigen specific editing of the patient’s immune system.
Future Directions
Dr. Fairclough’s lab continues to develop its interests in AChR activation as well as the production of protein therapeutic agents that can modulate in an antigen specific manner:
- the immune systems of patients with autoimmune MG, and
- the function of mutant AChRs of slow channel congenital MG patients.

