Kyle D. Fink, Ph.D.
Brief Biography
 Dr. Fink is a neuroscientist with expertise in stem cell biology, rodent models of neurodegenerative diseases, cell transplantation, neuroimmunology, focusing specifically on the development of novel therapeutics for Huntington’s disease.
Dr. Fink is a neuroscientist with expertise in stem cell biology, rodent models of neurodegenerative diseases, cell transplantation, neuroimmunology, focusing specifically on the development of novel therapeutics for Huntington’s disease.
During his graduate studies at Central Michigan University and the University of Nantes, Dr. Fink studied the therapeutic benefit of adult stem cell transplantation on the functional and neuropathological deficits in diseases of aging. These studies mainly focuses on the uses of mesenchymal stem cells and induced pluripotent stem cell transplantation into the striatum of mouse and rat models of Huntington’s disease.
During his postdoctoral training at the University of California, Davis Medical Center, he continued his work with engineered mesenchymal stem cell transplantation into Huntington’s disease mice as part of IND-enabling studies aimed at a Phase I clinical trial.
Because of this interest in Huntington’s disease, Dr. Fink obtained an NIH postdoctoral fellowship to pursued gene editing strategies to be used in conjunction with stem cell transplantation. Dr. Fink is now an Adjunct Assistant Professor in the Department of Neurology and is continuing his work in the UC Davis Stem Cell Program investigating the therapeutic potential of targeted epigenetic modification for Huntington's disease and other central nervous system disorders.
Research Overview
Dr. Fink's research aims to develop new therapies for neurodegenerative diseases, brain injury, and some forms of brain cancer. His research team focuses on genetically-linked neurological disorders such as CDKL5 deficiency (rare intractable pediatric epilepsy), Angelman’s Syndrome, and Huntington’s disease that are targetable with gene editing molecules in addition to some forms of brain cancer.
His lab is identifying candidate sequences through whole genome sequencing and transcriptomics using a bioinformatics approach for structural variation discovery and genotyping that identify underlying common genetic variants. Gene variants linked to disease phenotype are examined for “actionable domains” for which we create therapeutic artificial transcription factors. Patient-derived samples can be used to create in vitro neuronal disease-in-a-dish models using novel induced neuron techniques.
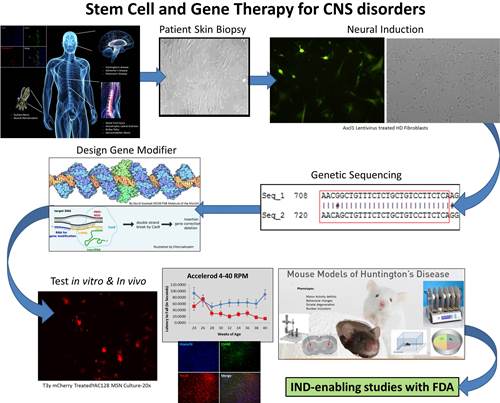
His team builds on the existing strengths and expertise at UC Davis and utilizes the necessary resources to develop a therapeutic pipeline in which precision medicine can be used to identify novel disease causing genetic variants to advance Precision Neurotherapeutics to clinical applications.
Research Program Details
Dr. Fink's current research focuses on two major goals: 1) The therapeutic application of transcription activator-like effector (TALE) and clustered regularly interspaced short palindrome repeats (CRISPR) to modify gene expression in genetically-linked pediatric neurological diseases; and 2) the development of delivery systems that can safely and efficaciously target the central nervous system with our novel gene editing platforms.
These goals specifically address: A) how to deliver these potent therapeutics to maximize the biodistribution in the central nervous system; B) how to increase the specificity of each construct to limit off-target effects; and C) how to minimize the immune response to increase safety and the therapeutic potential of this approach.
His team has used known information regarding Juvenile Huntington's disease (JHD) and CDKL5 deficiency to guide construction of potent, precise gene modifying modalities – CRISPR/Cas9 and TALE. These platforms can turn on or enhance transcription of a gene, transcriptionally silence expression, cause permanent epigenetic changes, or remove a piece of DNA via targeted double stranded breaks essentially allowing for custom modifications to be made at the genomic level.
TALE
- Derived from plant pathogenic bacteria from the genus Xanthomas
- One of many DNA-targeting proteins
- Each repeat comprises 33-35 amino acids
- Can be rapidly synthesized to target any base pair sequence
- Highly efficient and specific with minimal off-target effects
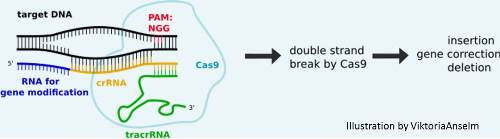
CRISPR/Cas9
- Original Cas9 derived from Streptococcus pyogenes
- Uses a synthetic guide RNA (gRNA) to deliver the Cas9 to a desired location
- Cas9 can be used interchangeably with different gRNA allowing for rapid targeting and flexibility
Both systems can be constructed with a variety of transcription factors to epigenetically regulate gene expression (i.e., nucleases, activators, repressors).
For a graphic summary of Dr. Fink's current research into potential gene modification treatment for Huntington's disease, please see Targeted Epigenetic Modification (PDF).
Future Directions
Continuing development of TALE, CRISPR, and other therapeutic modalities and delivery systems for other disease indications utilizing two complementary approaches: 1) Gene activation for some diseases characterized by gene loss of function, such as Angelman's Syndrome and infantile epilepsy; and 2) Gene silencing for gain-of-function diseases and disorders, such as Huntington's disease and genes that may be implicated in cancer.

