Fredric Gorin, M.D., Ph.D.
Brief Biography
 Dr. Gorin is a board certified neurologist with a doctorate in Biophysics and Physiology trained in computer-based small molecule drug-design and more recently, cancer biology. He received his undergraduate degree in Biochemistry from UC Davis and a combined medical and doctoral degree at the Washington University Medical School in St. Louis, Mo.
Dr. Gorin is a board certified neurologist with a doctorate in Biophysics and Physiology trained in computer-based small molecule drug-design and more recently, cancer biology. He received his undergraduate degree in Biochemistry from UC Davis and a combined medical and doctoral degree at the Washington University Medical School in St. Louis, Mo.
Dr. Gorin’s doctoral research generated pharmacophore that predicted how brain opioid peptides bind to the mu opioid receptor. At UCSF, he cloned and chromosomally mapped the first human muscular disorder (McArdle’s disease). At UC Davis his lab identified how neural activity coordinately regulates glycolytic metabolic gene expression in skeletal muscle. Current research has resulted in several patents for novel small-molecule therapies designed to kill hypoxically reprogrammed cancer cells that cause recurrence in aggressive cancers including brain cancers and metastatic cancers, notably breast, pancreatic, and lung.
Dr. Gorin oversees a nationally recognized Neurology department with more than 100 faculty, volunteer clinical faculty, post-doctoral fellows, residents, graduate students, clinical fellows and staff members, who conduct research and provide expert clinical and diagnostic services for individuals suffering from a broad array of neurological and neuromuscular conditions, including Alzheimer's and other neurocognitive disorders, epilepsy, cerebrovascular disease, Huntington’s, Parkinson's, traumatic brain injury, ALS and additional neuromuscular disorders.
Research Overview
High grade gliomas (HGGs) are very lethal cancers of the CNS, accounting for 78% of adult onset primary central nervous system malignancies. The 5-year survival for patients with glioblastoma multiforme receiving conventional therapy is approximately 9%. Malignant gliomas recur in more than 90% of patients despite radiation therapy, chemotherapy, or treated with anti-angiogenic agents, most notably bevacizumab. The high incidence of recurrence is a consequence of a sub-populations of hypoxically reprogrammed cancer cells that survive current therapies and are capable of tumor regrowth. These glioma-initiating cells possess several properties of normal pluripotent stem cells, including self-aggregation, renewal, the expression of stem cell markers.
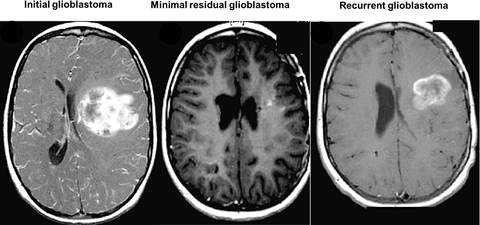
Therefore, designing and developing therapeutics targeting glioma “stem-like” cells (GSCs) is critical in the treatment and eradication of glioblastoma.
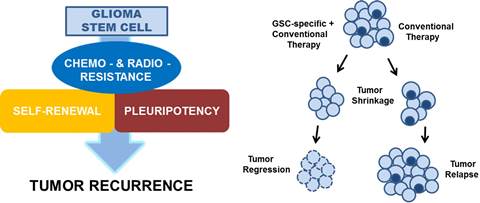
We have designed and synthesized novel small molecules that kill proliferating and non-proliferating hypoxically reprogrammed glioma cells within HGG cell lines and from 16 patient-derived xenografted glioblastomas. These cell permeant small molecules have been shown to irreversibly kill glioblastoma and breast cancer cells by a novel programmed irreversible necrotic glioma cell death mechanism mediated by apoptotic inducible factor (AIF). The parent compound UCD38B is patented by UC Regents.
Research Program Details
Research in our laboratory focuses on understanding how our new anti-cancer small molecules kill GSCs that cause cancer recurrence. Protein levels of intracellular uPA and PAI-1 are elevated in several malignant cancer cell types, including HGGs and metastatic breast cancers, and are predictive for solid cancers that are highly prone to proliferate, invade, recur and metastasize.
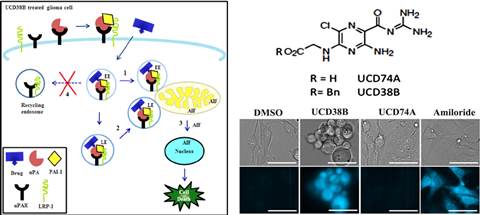
The patented parent compound, UCD38B, and its lead small molecules preferentially kill HGG cells residing within avascular perinecrotic regions and hypovascularized tumor margins of human glioma xenografts implanted intracerebrally in immunodeficient (NGS) mice.
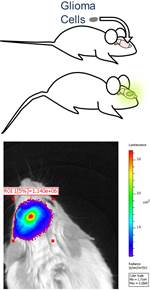
- Human glioblastoma cells are stably transfected to express luciferase and then intracranially implanted into immune deficient mice (NOD scid gamma).
- The transfected glioma cells bioluminesce when luciferin is administered to the mouse.
- Intracranial tumor volume is quantified by measuring bioluminescence intensity (IVIS Spectrum at CMGI).
Future Directions
Identify the in vivo pharmacological properties and efficacies of new anti-glioma small molecules to treat intracerebral PDX-HGG tumors.
We envision that the novel small molecules, in combination with conventional anti-cancer therapies, has the potential to reduce the risk of cancer recurrence and metastasis.

